ID: PMRREP3044| 165 Pages | 30 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

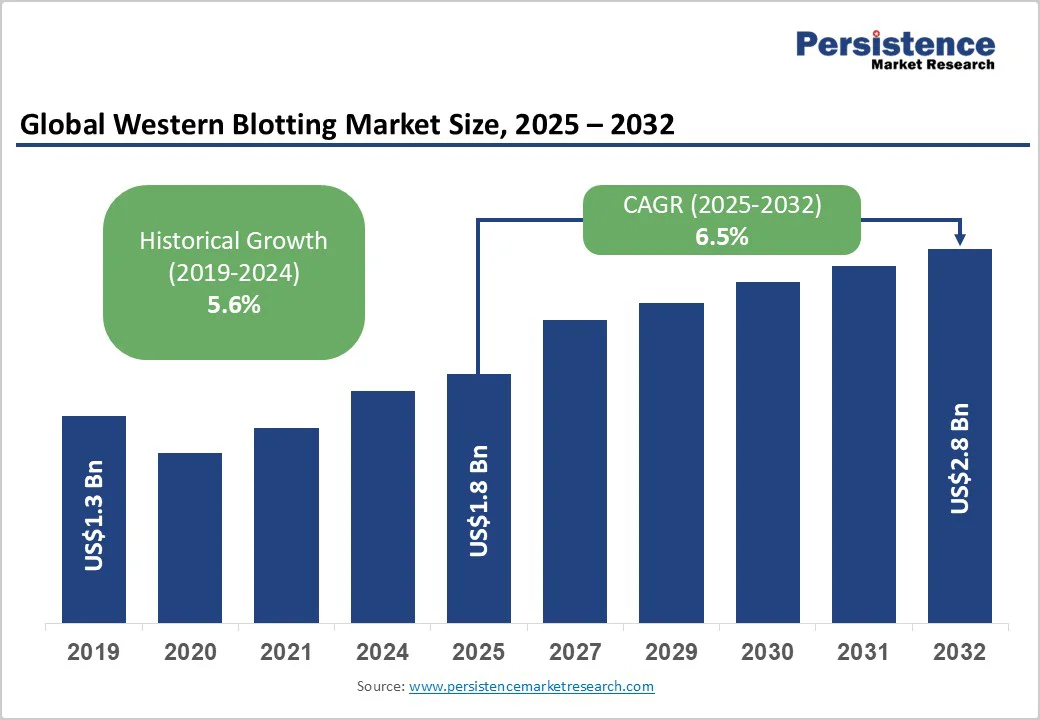
The global western blotting market is expected to reach US$1.8 billion in 2025. It is estimated to reach US$2.8 billion in 2032, growing at a CAGR of 6.5% during the forecast period 2025 - 2032, driven by the increasing prevalence of chronic conditions such as cancer, Alzheimer’s, and autoimmune disorders. It has pushed the requirement for accurate detection of disease-related biomarkers.
| Key Insights | Details |
|---|---|
| Western Blotting Market Size (2025E) | US$1.8 Bn |
| Market Value Forecast (2032F) | US$2.8 Bn |
| Projected Growth (CAGR 2025 to 2032) | 6.5% |
| Historical Market Growth (CAGR 2019 to 2024) | 5.6% |
The increasing global burden of chronic diseases such as cancer, diabetes, and neurodegenerative disorders has boosted the demand for accurate protein detection and quantification tools, including western blotting. Researchers depend on this technique to study disease-related protein markers and signaling pathways involved in disease progression.
For example, in 2025, studies from the National Cancer Institute used western blotting to confirm protein-level changes in tumor suppressor pathways during targeted therapy trials. As chronic illnesses account for over 70% of global deaths, labs are increasingly relying on unique blotting platforms to validate molecular findings that can aid in early diagnosis and treatment planning.
The ongoing expansion of proteomics research and biomarker discovery is fueling the use of western blotting as a validation step after mass spectrometry or omics-based screening. Pharmaceutical companies and academic labs use the technique to confirm potential therapeutic targets and verify differential protein expressions.
In 2024, Europe-based research consortia under Horizon Europe integrated automated blotting into biomarker workflows for Alzheimer’s and cardiovascular studies, improving throughput and reproducibility. As global funding for proteomic projects continues to rise, western blotting remains central to translating molecular insights into clinical applications by confirming protein-level authenticity and biological relevance.
The growing use of alternative detection technologies, such as ELISA, AlphaLISA, and multiplex bead-based assays, is limiting the expansion of traditional western blotting. These new platforms provide quick turnaround times, minimal manual handling, and high scalability for high-throughput screening, which are beneficial in drug discovery and diagnostic applications.
For instance, PerkinElmer’s AlphaLISA technology, widely adopted in 2024 for protein-protein interaction studies, enables homogeneous assays without the requirement for washing or blotting steps. The shift toward such automated, cost-efficient, and quantitative systems is gradually replacing western blotting in applications where precision and speed outweigh visual confirmation.
Western blotting remains resource-intensive due to the high initial cost of imaging instruments and the recurring expense of quality antibodies and reagents. Unique fluorescence imagers and automated capillary systems, including Bio-Techne’s Simple Western or LI-COR’s Odyssey, require significant investment and regular calibration.
Validated primary antibodies can also cost several hundred dollars per target, making large-scale studies expensive for small labs. The requirement for high-quality consumables and experienced personnel adds to operational overhead. Several academic institutions and start-ups in 2025 are turning toward shared core facilities or alternative assays to reduce financial strain while maintaining research output.
The development of automated and microfluidic-based western blotting systems is creating new opportunities for reproducible and high-throughput protein analysis. These platforms minimize manual errors, reduce reagent use, and allow parallel processing of multiple samples, making them ideal for large-scale proteomic studies.
Several biotech start-ups in the U.S. and Japan are developing chip-based blotting systems that integrate electrophoresis and imaging on a single platform. Such developments are expected to make protein profiling more efficient and accessible to mid-sized labs and diagnostic facilities.
Regulatory agencies and funding bodies are pushing for high standards of data reproducibility in biomedical research, which is opening doors for standardized Western blotting solutions. The U.S. National Institutes of Health (NIH) and the European Research Council (ERC) now require strict validation of protein data in grant-funded studies.
Automated blotting systems with digital documentation and traceable software, including Thermo Fisher’s iBright and LI-COR’s Image Studio, comply with these regulatory expectations. In 2025, several labs in Europe involved in Horizon Europe programs upgraded to automated blotting systems to comply with reproducibility mandates. This regulatory shift is encouraging the adoption of validated and quantifiable blotting technologies that ensure consistent and transparent protein data.
Consumables are expected to capture approximately 66.3% of the market in 2025, as membranes, buffers, and antibodies are frequently replaced during western blotting runs, driving demand. Unlike instruments, these items are used up with each experiment, and variations in quality directly affect data accuracy.
Researchers also prefer branded and pre-validated consumables from companies such as Abcam and Merck to ensure consistent protein detection. The surging shift toward fluorescence-based detection has increased the use of specialized secondary antibodies and substrates.
The steady growth in instruments comes from the gradual replacement of manual blotting setups with automated or semi-automated systems. Labs are now investing in imaging and analysis instruments that provide quick, digital, and reproducible results.
For example, LI-COR’s Odyssey systems are gaining popularity for their multiplex fluorescence detection and data traceability features. The trend toward integrated workflows, in which a single instrument handles blotting, imaging, and quantification, continues to attract institutional buyers despite high initial costs.
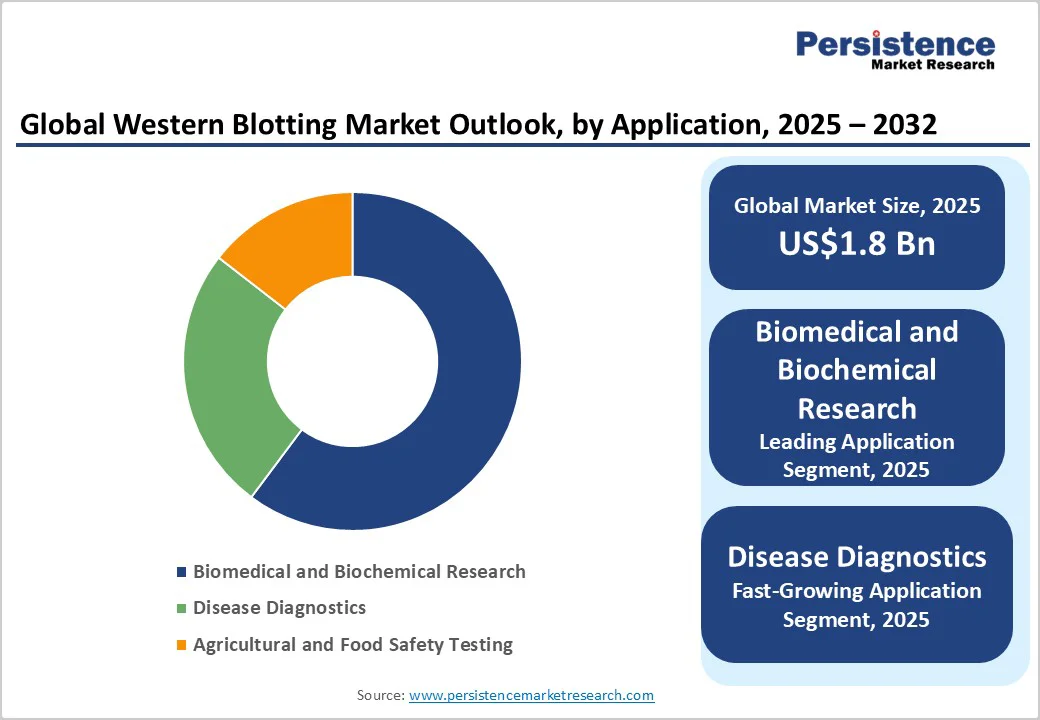
Biomedical and biochemical research will likely account for about 60.2% of the market in 2025, as western blotting remains indispensable for studying protein expression, phosphorylation, and post-translational modifications. Researchers use it extensively to validate proteomic and genomic data, especially in fields such as cancer, neuroscience, and cell signaling. For instance, recent Alzheimer’s studies published in 2025 utilized western blotting to confirm differential tau protein expression detected through mass spectrometry.
Western blotting is important in confirming infectious and autoimmune diseases due to its high specificity. It is commonly used as a confirmatory test for conditions such as HIV and Lyme disease, where enzyme immunoassays provide preliminary screening.
The CDC still recommends Western blot or immunoblot confirmation for certain serological tests, reinforcing its diagnostic relevance. Recent developments include integrating chemiluminescent and capillary-based blotting into diagnostic labs to improve sensitivity in low-sample analyses.
Academic and research institutes are poised to account for nearly 48.6% of the share in 2025, since western blotting remains a core method in basic life science education and discovery research. These institutions conduct thousands of experiments weekly to study signaling pathways, protein interactions, and molecular responses. With increasing funding for proteomics and structural biology in the U.S., Europe, and Japan, demand from academic settings continues to rise.
Hospitals and diagnostic labs rely on western blotting for confirmatory disease testing and for validating novel diagnostic assays. Clinical microbiology and immunology departments use blotting to confirm uncertain ELISA or PCR results, especially in infectious disease diagnosis.
For example, Western blot tests remain part of confirmatory HIV testing algorithms in several regions, including the Asia Pacific and Africa. Also, pathology labs extensively employ semi-automated blotting systems for protein profiling in cancer diagnostics.
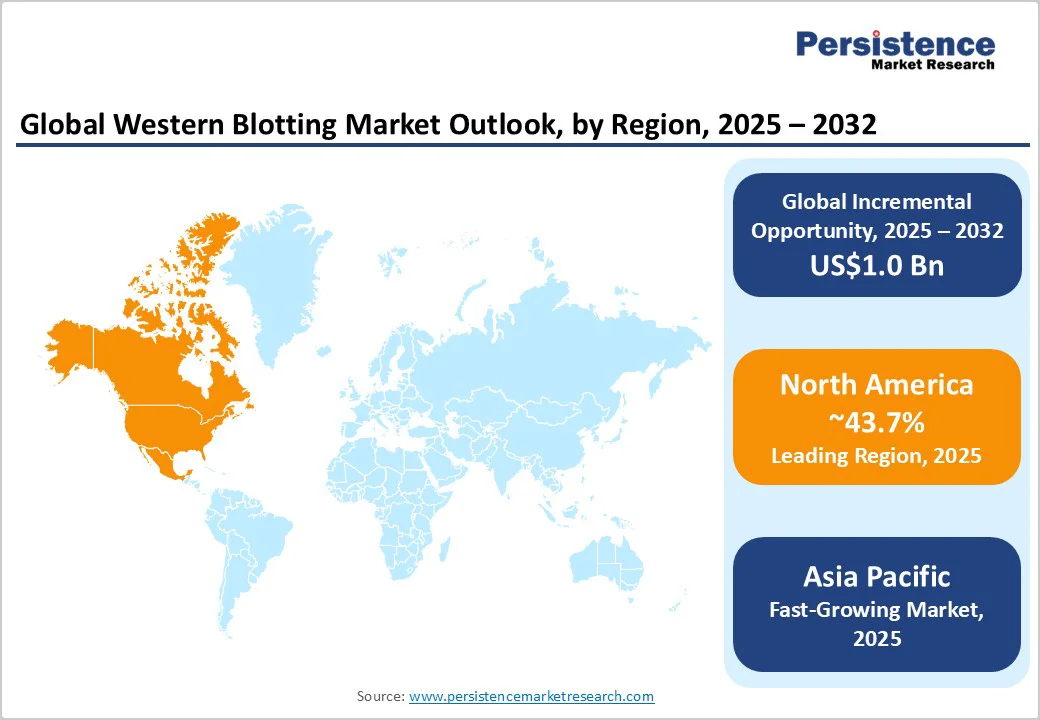
North America is anticipated to account for approximately 43.7% of share in 2025, owing to novel research infrastructure and heavy investment in biotechnology and pharmaceutical research and development. The U.S. leads the region, with widespread adoption of automated and digital blotting platforms across university labs, diagnostic centers, and biopharma companies.
Institutions such as the NIH and leading universities are now prioritizing quantitative and reproducible protein analysis, which has increased demand for systems, including Bio-Techne’s Simple Western and Thermo Fisher’s iBright series.
The market is also benefiting from government-supported cancer and neurobiology studies that rely on western blotting for biomarker validation. Also, collaborations between equipment makers and academic research centers, such as the 2024 partnership between Bio-Rad and Stanford’s Protein Sciences Division, are driving innovation in automated blotting workflows.
In Asia Pacific, the western blotting scenario is evolving rapidly as regional biotech and academic research capacity expand. China, India, Japan, and South Korea are investing heavily in life sciences and diagnostics, which has fueled demand for reliable yet cost-effective blotting systems. Various Asia Pacific-based labs still use conventional blotting methods due to their low cost. However, there is a growing shift toward capillary-based and digital systems for high-throughput and quantification.
In 2025, several China- and Korea-based biotech start-ups have begun integrating automated blot systems to improve reproducibility in clinical protein studies. Asia Pacific’s focus on infectious disease research, vaccine development, and cell signaling studies has further strengthened its demand. Local reagent suppliers in China and India are also entering partnerships with global companies, including Merck and Abcam, to distribute antibodies customized for regional applications.
In Europe, western blotting remains a key tool in protein research, particularly in molecular biology, immunology, and neurodegenerative disease research. Domestic laboratories focus heavily on reproducibility, compliance with data integrity standards, and sustainability. Germany, the U.K., and France are leading adopters of digital and multiplex imaging systems such as LI-COR’s Odyssey range and Bio-Rad’s ChemiDoc Go, which enable precise, multi-protein detection.
The European Union’s strict regulatory standards for diagnostics and therapeutic development have encouraged the use of automated blotting platforms that deliver traceability and electronic data storage. Also, EU-funded initiatives promoting open-access proteomic research, including Horizon Europe projects launched in 2024, are augmenting collaboration among research institutes and equipment manufacturers to develop standardized western blotting workflows.
The global western blotting market is dominated by established life science companies, including Thermo Fisher Scientific, Merck KGaA, Danaher (through Cytiva), Bio-Rad Laboratories, and Bio-Techne.
These players maintain their lead by delivering complete workflow solutions, right from sample preparation kits and antibodies to imaging instruments and analysis software. Their wide product portfolios and superior distribution networks give them a competitive edge, especially in academic and pharmaceutical research sectors where reliability and standardization are key.
The western blotting market is projected to reach US$1.8 Billion in 2025.
The rising prevalence of chronic diseases and the surging shift toward automated systems are the key market drivers.
The western blotting market is poised to witness a CAGR of 6.5% from 2025 to 2032.
Increasing focus on reproducible protein data and adoption of microfluidic blotting platforms are the key market opportunities.
Bio-Rad Laboratories, Thermo Fisher Scientific, and Merck KGaA (Millipore Sigma) are a few key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author