ID: PMRREP35097| 192 Pages | 15 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

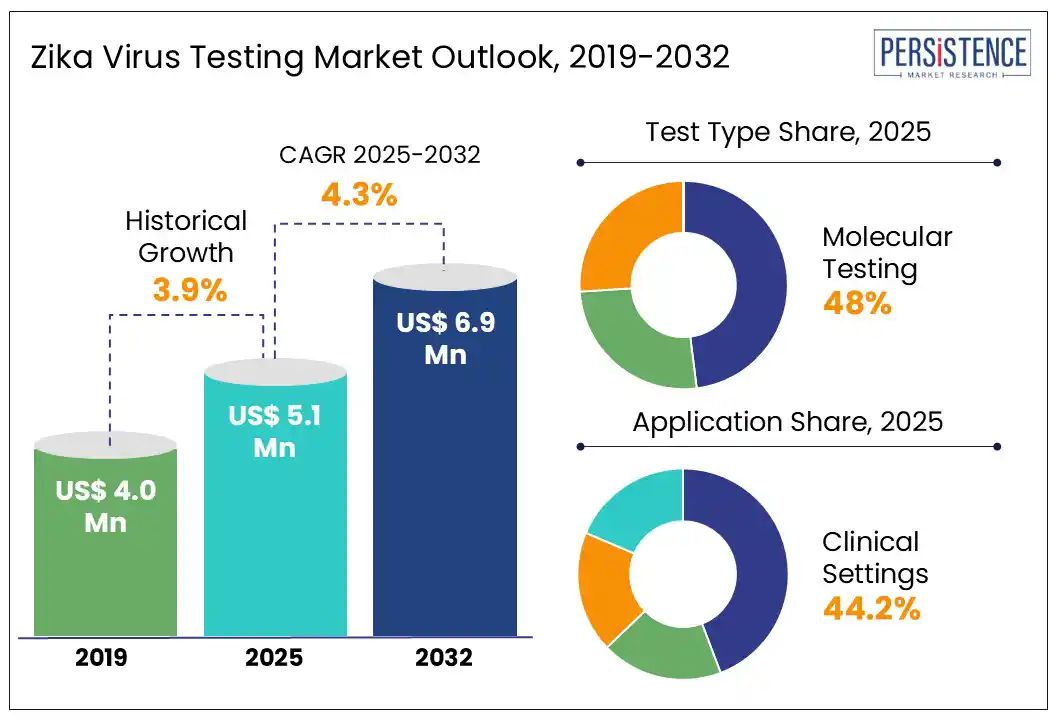
The global Zika virus testing market size is likely to be valued at US$ 5.1 Mn in 2025 and is estimated to reach US$ 6.9 Mn in 2032, at a CAGR of 4.3% during the forecast period 2025 - 2032.
Zika virus is a mosquito-borne infection primarily transmitted by Aedes mosquitoes, with additional risks of sexual and maternal-fetal transmission. Although often mild or asymptomatic, the virus gained global concern due to its link to neurological complications and severe birth defects during the 2015–2016 outbreak. The outbreak of the disease has produced significant growth opportunities for the Zika virus testing market, with molecular diagnostics such as RT-PCR gaining preference for early and accurate detection. Therefore, the zika virus testing market growth is driven by the rising prevalence of outbreaks, increased public health awareness, technological advancements in diagnostics, and supportive government policies for disease containment.
Serological tests also remain vital despite complications and challenges such as cross-reactivity. Hospitals and clinics account for the majority of testing due to superior infrastructure, while growing investment in point-of-care diagnostics and multiplex platforms is expanding access across resource-limited areas. Strong government support and rising public health surveillance are expected to fuel the market expansion.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Zika Virus Testing Market Size (2025E) |
US$ 5.1 Mn |
|
Market Value Forecast (2032F) |
US$ 6.9 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
4.3% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.9% |
The recurrence of Zika virus outbreaks across several parts of the world, such as the one experienced in India in 2024, has significantly heightened the demand for reliable diagnostic tools, propelling the Zika virus testing market growth. Countries across Latin America, Asia Pacific, and parts of North America continue to report periodic cases, keeping public health systems on alert. This ongoing threat is encouraging governments and healthcare institutions to invest in early detection methods, particularly molecular testing technologies such as RT-PCR, which offer high sensitivity during the early phase of infection. The push for accurate and rapid Zika virus testing technologies is further supported by increased awareness and surveillance initiatives, making diagnostic testing instrumental in containing epidemics in Zika-prone regions.
Despite their widespread use, serological tests in the Zika virus diagnostics face limitations due to the probability of cross-reactivity with related flaviviruses such as dengue. This overlap makes it difficult to distinguish between infections, especially in endemic areas where co-infection risks are high. Such ambiguity in diagnostic results can lead to underreporting or misclassification of Zika cases, undermining timely public health responses. While molecular assays offer better specificity, their limited availability in several low-resource environments creates a persistent barrier to accurate diagnosis and slows overall market growth.
Promising advancements in diagnostic technology are opening new avenues in the Zika virus testing market, especially in the case of point-of-care testing. Companies are increasingly developing tools that can simultaneously detect multiple mosquito-borne infections, including Zika, dengue, and chikungunya, using a single platform. These solutions are particularly valuable in regions where rapid differentiation between co-circulating viruses is crucial. Government funding and global health initiatives are further supporting the development and deployment of such innovations, making advanced diagnostics more accessible. As testing becomes more integrated, portable, and precise, the market is set to amplify its reach across both high- and low-income countries.
By test type, the Zika virus testing market has been bifurcated into molecular testing and serological testing. The molecular testing segment is projected to hold a leading market share of 48% in 2025, owing to its ability to deliver highly accurate results and early detection capabilities. Technologies such as RT-PCR and NAATs are widely used to identify Zika infections in the initial stages, making them essential tools during outbreaks. Moreover, these tests help reduce the risk of false positives, which is a common feature in serological methods, and therefore preferred in clinical settings. With continued emphasis on effective outbreak response and timely and precise diagnosis, molecular testing remains a critical component of the Zika diagnostics market.
Based on application, this market has been divided into clinical testing, blood screening, research and development, and prenatal testing. Out of these, the clinical testing segment is anticipated to account for the largest share at 44.2% in 2025, with hospitals and diagnostic laboratories serving as primary centers for sample collection and processing. These facilities are equipped with the necessary infrastructure and expertise required for accurate molecular and serological testing. Hospitals play an important role in regions facing active or recurring outbreaks. As awareness grows and public health systems evolve, clinical settings will continue to be the bedrock of Zika virus testing across the globe.
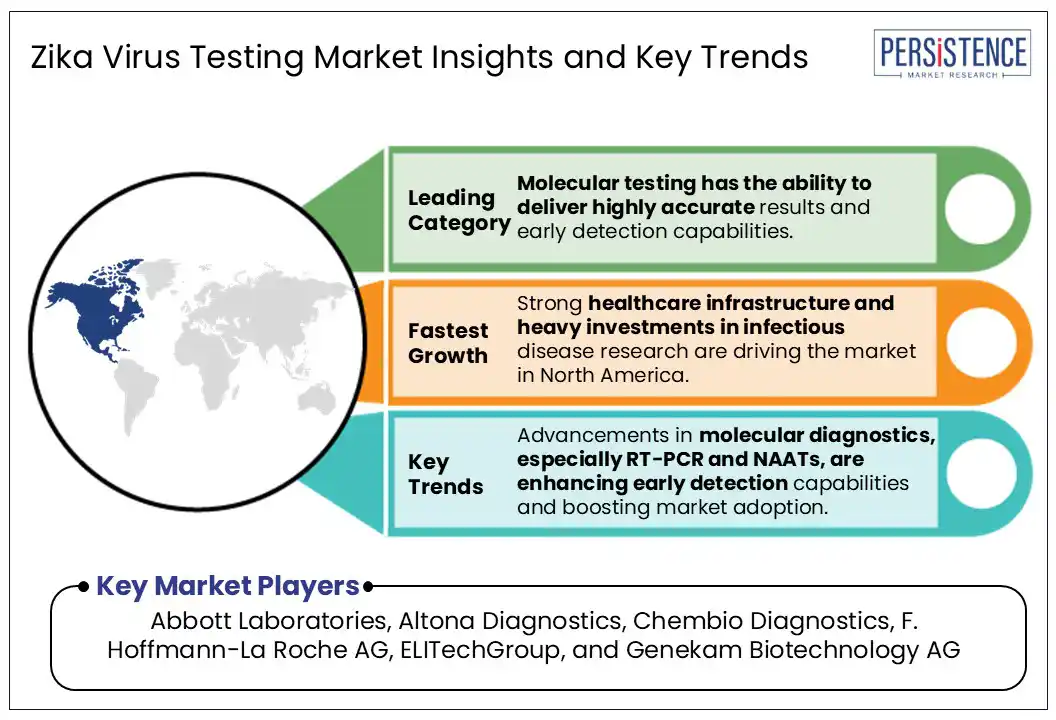
North America, accounting for 42.4% of the Zika virus testing market share, is expected to retain its dominant position through 2032. This is mainly attributable to the region’s strong healthcare infrastructure and heavy investments in infectious disease research. The region’s advanced diagnostic settings increasingly rely on molecular testing techniques such as RT PCR and NAATs, valued for their precision in detecting early-stage Zika infections. The rise of digital health platforms and ecommerce procurement channels has made test kits more accessible to both healthcare professionals as well as patients. Driven by a heightened outbreak awareness and sustained public health surveillance, North America’s leadership position as a research and diagnostic hub in the Zika virus testing space is set to remain constant in the forthcoming years.
Asia Pacific is likely to emerge as a high-growth region in the Zika virus testing market, backed by rising incidence of mosquito-borne infections and increasing awareness around early disease detection. Countries across the region are enhancing their diagnostic capabilities, particularly by adopting molecular testing methods for improved accuracy in identifying Zika infections. As governments strengthen public health systems, testing infrastructure, and surveillance programs, the demand for rapid and reliable Zika virus testing is likely to spike in the near future. Besides this, the expansion of clinical laboratories and greater availability of test kits are supporting the region’s expanding role in the Zika diagnostics landscape.
The global Zika virus testing market is characterized by a highly competitive landscape, with several global and regional players focusing on technological innovation and new product development. Companies are investing in advanced molecular diagnostics, such as NAAT-based platforms, to improve detection accuracy and reduce turnaround times. Strategic collaborations, regulatory approvals, and expansion into emerging markets are some of the preferred approaches adopted by key players to strengthen their market presence. Moreover, with the growing demand for multiplex testing kits capable of detecting multiple arboviruses, manufacturers prefer primary research.
The market is projected to reach US$ 5.1 Mn in 2025.
The recurrence of Zika virus outbreaks across several parts of the world is the key market driver.
The market is poised to witness a CAGR of 4.3% from 2025 to 2032.
Innovative testing technologies are generating new opportunities in the market.
The major players in the Global Zika Virus Testing include Abbott Laboratories, Altona Diagnostics, and Chembio Diagnostics.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Test Type
By Application
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author