ID: PMRREP32746| 245 Pages | 15 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

The global viral vector vaccines market expanded at 19.5% CAGR from 2019 to 2024 and reached a size of US$ 829.4 Mn at the end of 2025. The market is forecasted to expand at a CAGR of 2.5% over the next 7 years to end up at US$ 985.9 Mn by 2032.
Viral vector vaccines are used to deliver genetic material or DNA via vaccines. This DNA is then transcribed by the recipient's host cells to elicit an immune response. To date, only 6 viral vector vaccines have been approved by the United States FDA for use in humans-two Ebola vaccines and four COVID-19 vaccines.
The Ebola virus can cause a severe and often deadly illness, which is referred to as Ebola virus disease (EVD). Since the first outbreak was reported in 1976, it has been a top priority to create an effective Ebola vaccine. The use of viral vector vaccines has emerged as a promising approach in the development of an Ebola vaccine. This market has experienced significant growth in recent years, mainly attributed to the rising incidence of Ebola virus disease (EVD) in Africa and other regions worldwide. Increased government and private sector investments in the research and production of Ebola vaccines are also fueling market growth.
Viral vector vaccines have demonstrated high efficacy rates in preventing infectious diseases, which has boosted demand for this type of vaccine. These vaccines have been found to have a better safety profile compared to some traditional vaccines, such as live attenuated vaccines. Viral vectors can be easily modified to express different antigens, which makes them ideal for developing vaccines against multiple diseases.
| Report Attribute | Details |
|---|---|
|
Viral Vector Vaccines Market Size (2025) |
US$ 829.4 Mn |
|
Projected Market Value (2032) |
US$ 985.9 Mn |
|
Global Market Growth Rate (2025 to 2032) |
2.5% CAGR |
|
Market Share of Top 4 Countries |
71.4% |
“Rising Incidence of Infectious Diseases across the World”
Increasing prevalence of infectious diseases, such as Ebola, Zika, and HIV, has necessitated the creation of more effective vaccines, which is boosting demand for viral vector vaccines. Emergence of pandemic-level diseases has led to increased procurement of vaccines by international organizations such as UNICEF.
UNICEF is working with national governments to ensure that countries can access affordable and high-quality vaccines in a timely manner. UNICEF is working on several initiatives to strengthen vaccine procurement to control the increasing spread of infectious diseases around the world. This is one of the key drivers of the viral vector vaccines market and will have a positive impact on the growth of this market in the coming future.
To satisfy this demand and ensure vaccine security, WHO is partnering with Global Alliance for Vaccine and Immunization, UNICEF, and others to develop a Global Ebola Vaccines Security Plan. This will require an enhanced capability for the supply of such vaccines and an increase in the number of manufacturers. This would eventually have a favorable effect on the market.
Government and non-government bodies are focusing on immunization campaigns to raise public awareness of vaccinations.
“Vaccination Hesitancy among Public and Stringent Safety Regulations”
Coordination among scientists, physicians, public health officials, industry and vaccine developers, and society is required for the development of new vaccines, which is a lengthy, methodical, expensive, and time-consuming process. To develop safe and effective vaccines that are widely used, these stakeholders must work together to overcome the aforementioned hurdles. The high cost of vaccine development is one of the biggest challenges experienced by several vaccine manufacturers. It costs about US$ 700 million to US$ 1 billion to develop a vaccine.
Vaccination hesitancy, rigorous safety regulations, societal demands for complete efficacy, rising requirements for single-dose efficacy, the urgency to respond promptly to global outbreaks, the limited number of vaccine manufacturers, the need for more complex vaccines to combat current pathogens, the low efficacy of some licensed vaccines, and the variability in the correlation between humoral immune responses and protection are among the common challenges faced in vaccine development. These challenges can impede the growth of the global market.
“Rising Awareness Regarding Ebola Vaccinations in the Region”
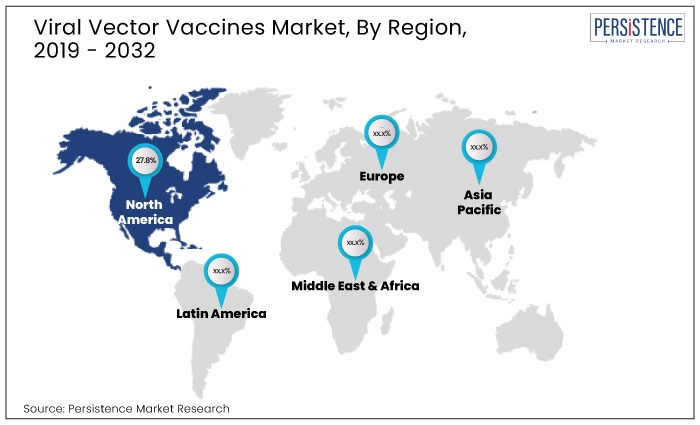
North Africa accounted for 27.8% share of the global market in 2024.
Recent Ebola virus disease outbreaks in West and Central Africa have highlighted the importance of developing effective vaccines to prevent the spread of the disease. This has resulted in a surge in demand for vaccines in the region, including North Africa.
Several governments in North Africa are taking initiatives to prevent the spread of infectious diseases such as Ebola. They are investing in vaccination programs to protect their populations from such diseases, which is expected to drive the growth of this market in North Africa. These drivers are expected to create a positive outlook for the growth of the viral vector vaccines market in North Africa in the coming years.
“Increased Government Funding for Vaccine Development”
The United States held 23.6% share of the global market in 2024.
The U.S. government has provided significant funding and support for the development of Ebola viral vector vaccines. This funding has enabled the development of new vaccines and the advancement of clinical trials, which is expected to drive the growth of the market. The threat of Ebola outbreaks and the potential for bioterrorism have led to increased emphasis on public health preparedness in the U.S. This has led to increased demand for effective vaccines against Ebola.
The United States has a highly advanced healthcare infrastructure, which enables the efficient and effective distribution of vaccines. This is expected to drive the growth of the viral vector vaccines market in the U.S.
“Increasing Collaborations between Pharmaceutical Companies and R&D Institutions for Ebola Vaccines”
South Africa held a share of 13.9% of the global market in 2024.
There has been an increase in research and development activities related to the development of viral vector vaccines against Ebola. This has resulted in the development of new vaccines that are more effective, easier to administer, and have fewer side effects. These developments are expected to drive the growth of the market in South Africa.
Increasing collaborations between pharmaceutical companies and research institutions to develop effective vaccines against Ebola. These collaborative efforts are expected to accelerate the development and commercialization of new vaccines and drive the growth of the market in South Africa.
“High Incidence of Ebola in Adult Population”
The adults segment held 64.5% share of the global market in 2024.
Ebola virus disease (EVD) can affect individuals of any age group, but studies have shown that it is more prevalent in adults than in pediatric populations.
It is important to note that children and adolescents have also been affected by these outbreaks, and they may have a higher risk of mortality. Adults may be more likely to be exposed to the virus through their work or caregiving roles, while children may have fewer opportunities for exposure.
“High Demand for Hospital Vaccination Services Due to Availability of Well-trained Medical Staff to Handle Adverse Reactions”
Hospitals held 67.5% of the global viral vector vaccines market in 2024.
Patients often prefer to get vaccinated from hospitals because hospitals are seen as trusted sources of medical care. Hospitals have a reputation for providing safe and effective medical treatment, and patients feel more comfortable receiving medical procedures in a hospital setting.
Hospitals also have the resources and expertise to handle any adverse reactions that may occur after vaccination. They have medical staff who are trained to recognize and treat adverse reactions, and they have the equipment and medications necessary to provide immediate care if needed. Hospitals often have convenient locations and flexible scheduling options, which can make it easier for patients to get vaccinated.
Many hospitals offer walk-in vaccination clinics or appointments that can be scheduled online or by phone, which can save patients time and hassle. All these factors make hospitals the dominant segment in the viral vector vaccines market.
Top pharmaceutical companies are focusing on regional development as a means of boosting revenue and expanding their sales presence in developing countries by acquiring local market players. To strengthen their market position globally and diversify their product portfolios, pharma giants are engaging in mergers and acquisitions.
Several notable examples of this trend include:
Similarly, the team at Persistence Market Research has tracked recent developments related to companies manufacturing viral vector vaccines, which are available in the full report.
The global viral vector vaccines market was valued at US$ 666.2 Mn in 2024.
Worldwide demand for viral vector vaccines rose at 19.5% CAGR from 2019 to 2024.
North Africa, the U.S., South Africa, and Türkiye hold 71.4% market share in 2025.
The United States accounted for 23.6% share of the global market in 2024.
North Africa accounted for 27.8% share of the global market in 2024.
South Africa occupied 13.9% of the global viral vector vaccines market share in 2024.
Adults accounted for 64.5% share of viral vector vaccinations in 2024.
Demand for viral vector vaccines is set to reach US$ 985.9 Mn by the end of 2032.
Sales of viral vector vaccines are projected to rise at a CAGR of 2.5% through 2032.
| Attribute | Details |
|---|---|
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2024 |
| Market Analysis Units | Value: US$ Bn/Mn, Volume: As applicable |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Covered |
|
| Report Highlights |
|
|
Customization & Pricing |
Available upon Request |
By Patient:
By Distribution Channel:
By Region:
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author