ID: PMRREP34774| 170 Pages | 14 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

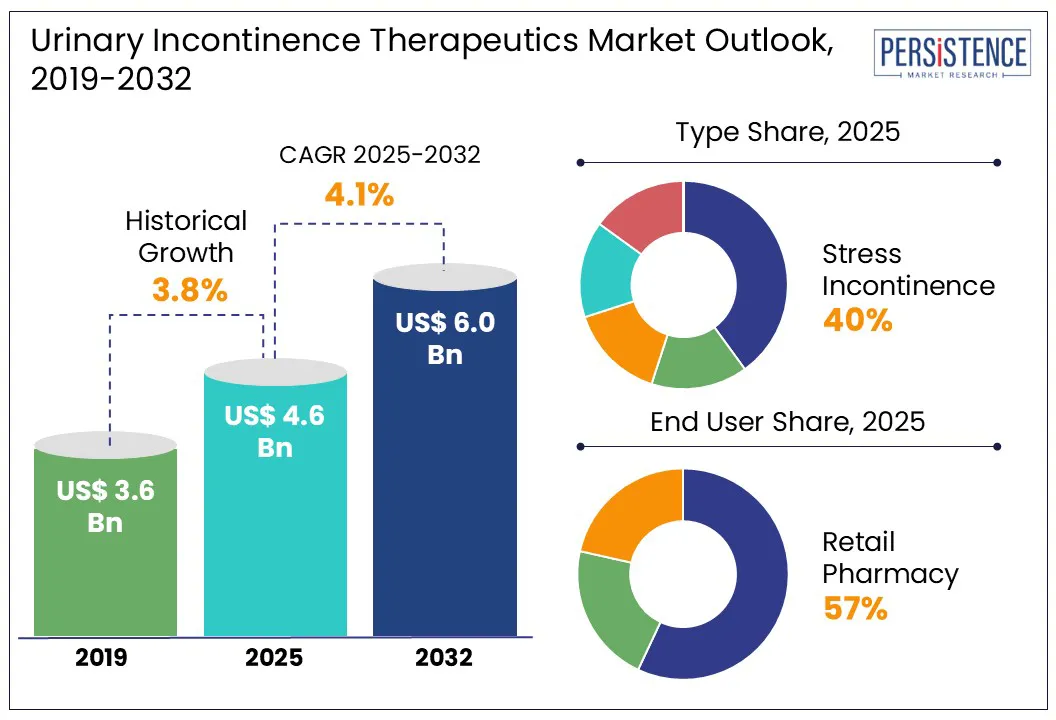
Global urinary incontinence therapeutics market size is likely to be valued at US$ 4.7 Bn in 2025 and is estimated to reach US$ 6.5 Bn by 2032, growing at a CAGR of 6.9% during the forecast period 2025−2032.
In 2025, the U.S. urinary incontinence therapeutics market captured a substantial portion of the North America market, driven by the widespread prevalence of the condition, which affects millions of adults.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Market Size (2025E) |
US$ 4.6 Bn |
|
Projected Market Value (2032F) |
US$ 6.0 Bn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
4.1% |
|
Historical Market Growth Rate (CAGR 2019 to 2024) |
3.8% |
|
Region |
Market Share in 2025 |
|
North America |
38.7% |
North America is projected to lead the urinary retention therapeutics market, capturing 38.7% of the market share by the end of 2025. North America leads the incontinence therapeutics market due to its aging population, high disease prevalence, and advanced healthcare infrastructure. In the U.S., around 22.1% of adult women about 28.5 million experience moderate to severe urinary incontinence. Prevalence increases with age, affecting over 35% of people aged 60 and older. The U.S. Census Bureau projects the 65+ population to reach 85 million by 2050. Innovation also drives growth, with FDA-approved drugs like GEMTESA and neuromodulation devices expanding treatment options.
Major companies such as Pfizer and AbbVie dominate the space, while strong insurance coverage and broad pharmacy access 57% of products are sold in pharmacies boost adoption. Over $500 million is invested annually in R&D.
|
Region |
Market Share in 2025 |
|
Europe |
27.5% |
Europe ranks among the leading regions in the incontinence therapeutics market due to its rapidly aging population, high disease burden, and strong healthcare infrastructure. In the European Union, more than 20.8% of the population was aged 65 or older in 2022, creating a substantial pool of individuals at risk for bladder-control issues
Community studies across France, Germany, Spain, and the UK report that approximately 35% of adult women experienced urinary leakage in the previous month, with prevalence as high as 44% in France and 42% in the UK . In nursing homes, rates range from 35.1% in Austria to over 80% of residents using absorbent products or catheters.
The economic impact is significant urgent urinary incontinence alone is estimated to cost €7?billion annually across Canada, Germany, Italy, Spain, Sweden, and the UK. These factors prevalence, aging demographics, institutional care needs, economic burden, and supportive policy drive Europe’s prominent role in the global incontinence therapeutics market.
|
Category |
Market Share in 2025 |
|
Type - Stress Incontinence |
40.6% |
By type of incontinence, the stress urinary incontinence segment is anticipated to hold a 40.6% market share by the end of 2025, leading the urine retention therapeutics market. The increase in stress urinary incontinence cases among women is the reason for the surge.
Approximately 50% of women who experience urine incontinence also report having stress incontinence symptoms, according to epidemiological studies on the condition in women. Research has also revealed that after prostate surgery, men's rates of stress urine incontinence vary from 2% to 70%. During the anticipated time, these variables are probably going to drive the segment's expansion.
|
Category |
Market Share in 2025 |
|
End User - Retail Pharmacy |
57.1% |
Retail pharmacy dominates the industry accounting for 57.1% market share in 2025. The retail pharmacies' extensive global reach and established market dominance are a result of their widespread availability and longevity.
Retail pharmacies provide convenience for people looking to purchase UI drugs because they are widely accessible to the public. This accessibility is essential particularly for elderly people who account for a large proportion of patients with incontinence. When patients are managing their use of over-the-counter (OTC) drugs and prescribed therapy, retail pharmacies are frequently their initial point of contact.
The increasing prevalence of urinary incontinence (UI) is due to the factors such as urinary tract infections, weakened pelvic floor muscles, pregnancy, childbirth, and post-surgery in men following prostate removal. These issues are expected to contribute to the rise of UI symptoms and fuel the growth of urinary retention therapies during the forecast period.
The increasing incidence of urological disorders and the development of advanced incontinence devices are projected to boost the urinary retention device market. The demand for minimally invasive procedures, along with an aging population more susceptible to urological issues, is also anticipated to drive market growth.
Many patients with urinary incontinence are shifting from traditional to advanced treatment methods benefiting market expansion. The increasing risk of related urological disorders is expected to further support the market's growth trajectory.
The increasing incidence particularly in older adults and post-surgical patients contributed significantly to market expansion. Development of minimally invasive treatments such as neuromodulation devices and botulinum toxin therapies gained traction in the past years. This has significantly contributed to the market's expansion. The market expanded at a CAGR of 3.8%, indicating steady growth during that period.
Development of minimally invasive treatments, such as neuromodulation devices and botulinum toxin therapies gained traction during this period. The market will benefit from a heightened focus on female urinary incontinence with companies investing in new products targeting women.
The urinary incontinence therapeutics market overview has shown consistent growth over the years, and its upward trajectory is expected to continue in the future. The market is projected to capture a CAGR of 4.1% during the forecast period from 2025 to 2032.
The rising number of prostate cancer cases is boosting the growth of the urinary incontinence therapeutics. market. There is also increasing demand for advanced, minimally invasive over-the-counter (OTC) devices and wearables, which is pushing research and development in urinary retention devices.
Medical technology companies are actively seeking FDA approval for their next-generation devices designed to treat stress incontinence, particularly in women. These developments are expected to fuel market growth in the coming years.
Rising awareness about urinary incontinence along with improved diagnostic methods is significantly driving the urinary incontinence therapeutics market. Public health campaigns and educational programs have led to increased knowledge about the condition encouraging more individuals to seek medical help.
With advanced diagnostic tools, healthcare providers can now identify and classify incontinence types more effectively leading to better-targeted treatments. This heightened awareness and diagnosis particularly among old adults and women are increasing the demand for both pharmacological and non-pharmacological therapies contributing to the overall growth of the market.
The urinary incontinence therapeutics market faces challenges due to a lack of awareness about new advancements in treatment options and the complications that can arise after using these devices.
The increasing cost of treatment and the vulnerability of the aging population are factors that reduce demand for urinary retention medications. The market's growth is further hindered by patients opting for alternative therapies. Another obstacle is the growing number of people with incontinence who are unaware of their condition, which limits the market's potential expansion during the forecast period.
The increasing cost of urinary incontinence therapies can make it difficult for patients to afford treatment. As the price of medications and devices rises, some individuals may hesitate to seek help or continue with prescribed treatments. This financial burden can lead to fewer people accessing the necessary care, which impacts their quality of life.
The high expense is a significant barrier preventing them from exploring or sticking with effective therapies, and ultimately limiting their treatment options and market growth for these products.
Innovative product development offers great opportunities for companies in the market. By focusing on new technologies like smart wearables, less invasive treatments, and customized therapies, companies can meet patient needs and stand out from competitors.
Creating advanced solutions with better results and few side effects can boost market growth. Investing in research to develop new products that cater to different patient needs can open new market segments and improve patient satisfaction leading to future success and leadership in the market.
There are many leading industry players in the fiercely competitive market. To treat urinary retention disease, these players are developing cutting-edge new delivery methods. The market for urinary incontinence therapeutics is anticipated to grow as a result.
Pfizer Inc., AbbVie Inc., Astellas Pharma Inc., Johnson & Johnson, Viatris Inc., and Teva Pharmaceutical Industries Ltd. are the leading companies in the market. Prominent industry participants tend to employ inorganic growth tactics such as partnerships, acquisitions, mergers, and collaborations to enhance their range of products. The global market for urinary retention therapies is expected to benefit from this.
By Type
By Drug Class
By End User
By Region
The Global market is estimated to increase from US$ 4.6 Bn in 2025 to US$ 6.0 Bn in 2032.
Rising aging population, increased prevalence of urinary disorders, growing awareness, and improved access to advanced therapies drive market growth.
The market is projected to record a CAGR of 4.1% during the forecast period from 2025 to 2032.
Opportunities include expanding in emerging markets, developing novel drug delivery methods, advancing neuromodulation therapies, and increasing telehealth-based incontinence management.
Major players include Pfizer Inc., AbbVie Inc., Astellas Pharma Inc., Johnson & Johnson, Viatris Inc., Teva Pharmaceutical Industries Ltd. and Others.
|
Attributes |
Details |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2024 |
|
Market Analysis |
US$ Billion for Value |
|
Key Regions Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author