ID: PMRREP35506| 194 Pages | 22 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

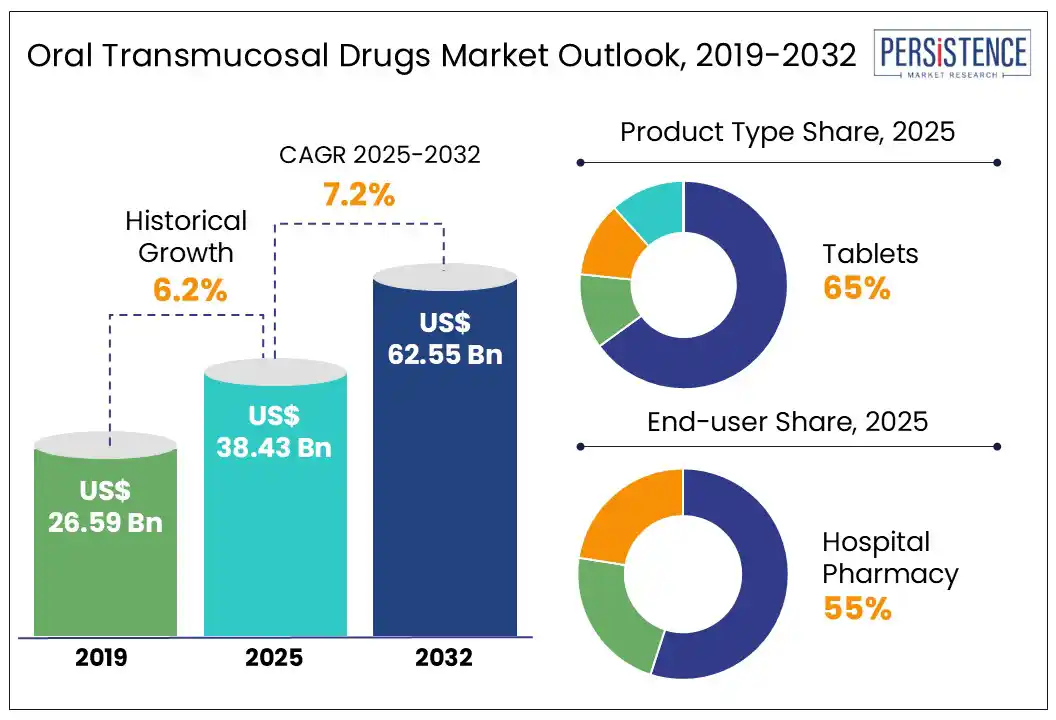
The global oral transmucosal drugs market size is likely to be valued at US$ 38.43 Bn in 2025 and is expected to reach US$ 62.55 Bn, growing at a CAGR of 7.2% during the forecast period from 2025 to 2032. Increasing demand for advanced drug delivery systems and innovative formulation techniques, driven by a global increase in disease burden, has led to market expansion over the forecast period.
Oral transmucosal drugs help in administering active pharmaceutical agents easily through the oral mucosa, bypassing the acidic stomach conditions and the enzymatic activity of the small intestine. It also helps in avoiding first-pass hepatic metabolism as the drug successfully penetrates the oral mucosa and reaches the bloodstream. It is particularly suitable for patients who have difficulty swallowing, such as pediatric and geriatric patients. Parenteral injections are expensive and often painful, requiring trained personnel for administration, while mucosal routes, such as ocular, oral (buccal/sublingual), nasal, and vaginal, offer alternatives by avoiding these limitations.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Oral Transmucosal Drugs Market Size (2025E) |
US$ 38.43 Bn |
|
Market Value Forecast (2032F) |
US$ 62.55 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.20% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.20% |
The increasing burden of chronic illnesses drives the oral transmucosal drugs market growth. Neurological disorders such as Parkinson’s disease affect nearly 10 million people globally. In the U.S., approximately 20.9% of adults suffer from chronic pain, which calls for fast-acting and non-invasive pain relief options. Oral transmucosal drug formulations, such as fentanyl buccal tablets and sublingual buprenorphine, are widely used in pain and addiction management. Chronic diseases, such as Parkinson’s disease, Alzheimer's, inflammatory bowel disease (IBD), and chronic pain conditions, require long-term medications that could be painful due to invasive methods of administration or gastrointestinal side effects. These routes enable rapid action while bypassing hepatic first-pass metabolism.
The use of mucoadhesive polymers, permeation enhancers, and enzyme inhibitors has significantly improved drug absorption through the oral mucosa. Moreover, technologies such as 3D-printed intraoral devices enable controlled-release formulations tailored to individual patient needs. Transmucosal routes, such as intranasal, buccal, sublingual, and rectal, have gained traction due to their non-invasive nature, ease of use, and the ease of self-administration or by caregivers. Oral transmucosal drugs, including sublingual and buccal films, patches, and sprays, have shown great promise through immediate action, better-quality bioavailability, and better convenience for patients.
Regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established stringent guidelines to ensure the safety, efficacy, and quality of oral transmucosal drugs. Ensuring dosage uniformity and content stability in films, sprays, and patches presents unique formulation challenges. Labeling and patient usage instructions for oral transmucosal drugs must be specific. Incorrect administration, such as swallowing instead of mucosal application, can result in reduced efficacy or unintended side effects.
For controlled substances such as opioids delivered transmucosally, additional risk evaluation and mitigation strategies (REMS) are authorized to avoid misuse, guaranteeing patient safety. Transmucosal immediate-release fentanyl medicines, such as Actiq and Fentora, are powerful opioids to treat breakthrough cancer pain and are subject to a strict FDA-mandated REMS. The modified REMS requires documented opioid tolerance for each prescription, pharmacy verification at every purchase, and patient enrollment in a national registry to ensure safe use. In June 2025, the U.S. FDA issued a Federal Register notice recommending labeling changes for buprenorphine-containing transmucosal products such as Suboxone and Zubsolv to address misinterpretations about maximum daily dosing. In September 2024, the FDA announced the discontinuation of all transmucosal immediate-release fentanyl medications, following Teva Pharmaceuticals' decision to stop the production of Actiq and Fentora.
R&D efforts have revolutionized oral transmucosal drugs, leading to the production of functionalized chitosan derivatives, a naturally-derived polymer modified with thiol, acrylate, or catechol to enhance mucoadhesion. These allow drugs to stick longer to the mucosal surfaces in the mouth, enabling better absorption and controlled release. Another notable trend in the transmucosal drug space is the use of nanocarrier-based systems. These nanocarriers penetrate mucosal barriers and increase drug residence time, thereby enhancing both efficacy and patient outcomes.
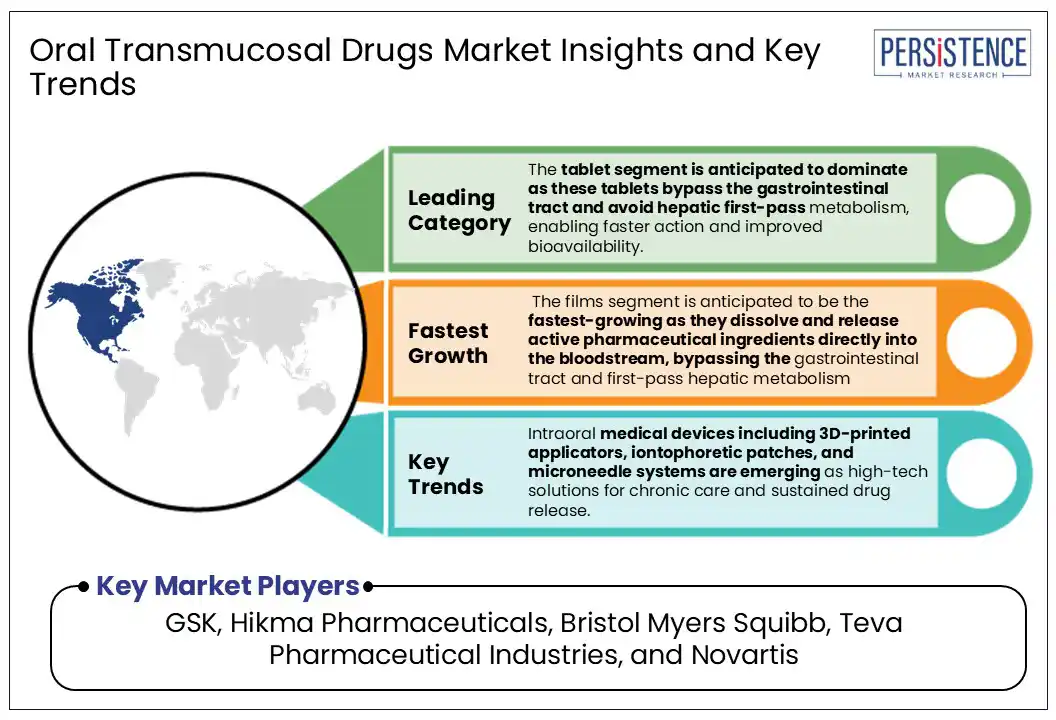
On the formulation front, fast-dissolving tablets (FDTs) and buccal films dissolve quickly in the mouth, and are ideal for people who have trouble swallowing pills, especially children and the elderly. Buccal films offer another user-friendly option, adhering to the inside of the cheek to deliver medication directly into the bloodstream while avoiding the digestive system entirely. Intraoral medical devices including 3D-printed applicators, iontophoretic patches, and microneedle systems are emerging as high-tech solutions for chronic care and sustained drug release.
The tablet segment is anticipated to dominate the market, capturing around 65% of revenue over the forecast period. These tablets are designed to dissolve directly in the oral cavity through the buccal (cheek) or sublingual (under the tongue) areas. Through oral mucosa, these tablets bypass the gastrointestinal tract and avoid hepatic first-pass metabolism, enabling faster action and improved bioavailability. This makes oral transmucosal tablets ideal in pain, seizures, anxiety attacks, and nausea requiring immediate attention. Fentanyl buccal tablets, such as Fentora®, are widely used in oncology for rapid pain relief. Oral transmucosal tablets are suitable for people with swallowing difficulties such as children, elderly patients, or those in palliative care.
The films segment is anticipated to be the fastest-growing segment over the forecast period. These ultra-thin and flexible buccal films are designed to stick to the inner lining of the mouth, where they dissolve and release active pharmaceutical ingredients directly into the bloodstream, bypassing the gastrointestinal tract and first-pass hepatic metabolism. A 2024 study published in Molecular Pharmaceutics demonstrated that incorporating glycerol into pectin-based films enhanced mucoadhesion and modulated drug release for paracetamol. Another innovative approach, featured in Drug Delivery and Translational Research, involved the use of nanostructured lipid carriers (NLCs) combined with 3D printing to produce cannabidiol buccal films with customizable drug release profiles. Buccal films are advantageous to children, the elderly, and patients in intensive care. They are discreet, require no water, and are easy to self-administer or use under caregiver supervision.
The hospital pharmacy segment is anticipated to dominate, accounting for 55% of the market revenue over the forecast period. This dominance is attributed to its critical role in dispensing medications to both inpatients and outpatients, particularly for patients with acute medical conditions requiring close monitoring and specialized pharmaceutical services. Their established infrastructure and direct access to a diverse patient population make them a vital component in the distribution of these drugs. Asia Pacific is witnessing rapid growth in healthcare infrastructure. For instance, Singapore is developing a 1,400-bed integrated hospital campus. In India, Apollo Hospitals plans to add over 2,000 beds by fiscal 2027.
The online pharmacies segment is expected to emerge as the fastest-growing over the forecast period, driven by increasing consumer demand for convenience, digital accessibility, and innovative service models. Patients can now order medications online with doorstep delivery, which is beneficial for people with mobility challenges or busy schedules. E-commerce platforms and digital health technologies, such as mobile apps, telehealth services, and automated refill systems, have streamlined the prescription process, making oral transmucosal drugs such as buccal fentanyl, sublingual lorazepam, and ondansetron orally disintegrating tablets (ODTs) more accessible. Companies, such as Amazon Pharmacy, CVS Health, and Walgreens, along with startups such as Strut and regional players including 1mg and PharmEasy, are leading this transformation.
North America dominates the market, holding over 45% share from 2025 to 2032, driven by an aging population and a rising prevalence of chronic and neurological conditions such as Parkinson’s disease, Alzheimer’s, and dysphagia. Regulatory support from both the U.S. Food and Drug Administration (FDA) and Health Canada provides detailed guidance on formulation standards, bioavailability assessments, and clinical trial protocols. Health Canada supports technologies such as Rapid Dose Therapeutics’ QuickStrip™. Key market players include BIAL, Tonix Pharmaceuticals, and Vestige Marketing.
The U.S. holds the largest share within the region, reinforced by its aging population, high chronic disease burden, and progressive regulatory environment. With over 76% of adults reporting at least one chronic condition in 2023, the demand for rapid & effective drug delivery options has surged. Conditions, such as opioid use disorder, diabetes, hypertension, and depression, remain highly prevalent.
The oral transmucosal drug market in Asia Pacific is witnessing rapid growth, fueled by demographic shifts, technological advancements, and evolving healthcare infrastructure. Countries including Japan and South Korea are leading in innovation, particularly in the development of dissolvable films and mucoadhesive drug delivery systems that improve bioavailability and patient adherence. With Japan’s geriatric population projected to reach 38% by 2050, the region faces rising demand for non-invasive therapies for age-related conditions such as Parkinson’s disease and dysphagia. Key pharmaceutical players, including C.L Pharm, Cure Pharmaceutical, and Seoul Pharmaceuticals, are driving investments in R&D. India’s Emcure and Lupin Limited are innovating in sublingual and buccal films using solvent-casting and hot-melt extrusion technologies for Central Nervous System (CNS) and pain management therapies.
China is the fastest-growing market, supported by healthcare infrastructure expansion and a large patient population. China’s rapidly growing burden of chronic diseases, including neurological disorders and pain-related conditions, further amplifies demand for fast-acting, non-invasive drug options. Leading domestic players such as Hubei New Desheng Material Technology and Shanghai Tenghu Biological Technology are gaining recognition for producing high-quality transmucosal formulations.
Europe is experiencing steady growth, fueled by an aging population and a rising prevalence of chronic diseases, particularly among elderly patients who may struggle with swallowing conventional tablets. European regulatory bodies are supporting this growth by providing favorable guidelines for clinical trials, safety, and efficacy standards. A notable example includes Aquestive Therapeutics’ collaboration with Pharmanovia to commercialize Libervant (diazepam buccal film) across the EU and UK. In Europe, DocMorris offers a broad range of oral transmucosal medications
France is emerging as the fastest-growing market within Europe for oral transmucosal drug delivery systems. This expansion is driven by a combination of increased healthcare spending, strong government support for pharmaceutical innovation, and a growing patient population with chronic and neurological disorders. The adoption of advanced medical technologies has encouraged wider use of non-invasive drug delivery systems, particularly among the aging population with dysphagia and neurodegenerative diseases.
The global oral transmucosal drugs market is highly competitive and fragmented, with numerous established global and regional players offering a wide range of products and vying for higher market share. Key players are focusing on developing innovative product solutions.
The global oral transmucosal drugs market size in 2025 is likely to be valued at US$ 38.43 bn.
The oral transmucosal drugs market is fueled by the increasing demand for advanced drug delivery systems and innovative formulation techniques due to a global increase in disease burden.
The oral transmucosal drugs market is poised to witness a CAGR of 7.2% from 2025 to 2032.
Intraoral medical devices including 3D-printed applicators, iontophoretic patches, and microneedle systems are emerging as high-tech solutions for chronic care and sustained drug release.
Major players in the Oral Transmucosal Drugs Market are GSK, Hikma Pharmaceuticals, BristolMyers Squibb, Teva Pharmaceutical Industries, and Novartis.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Route of Administration
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author