ID: PMRREP11908| 179 Pages | 1 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

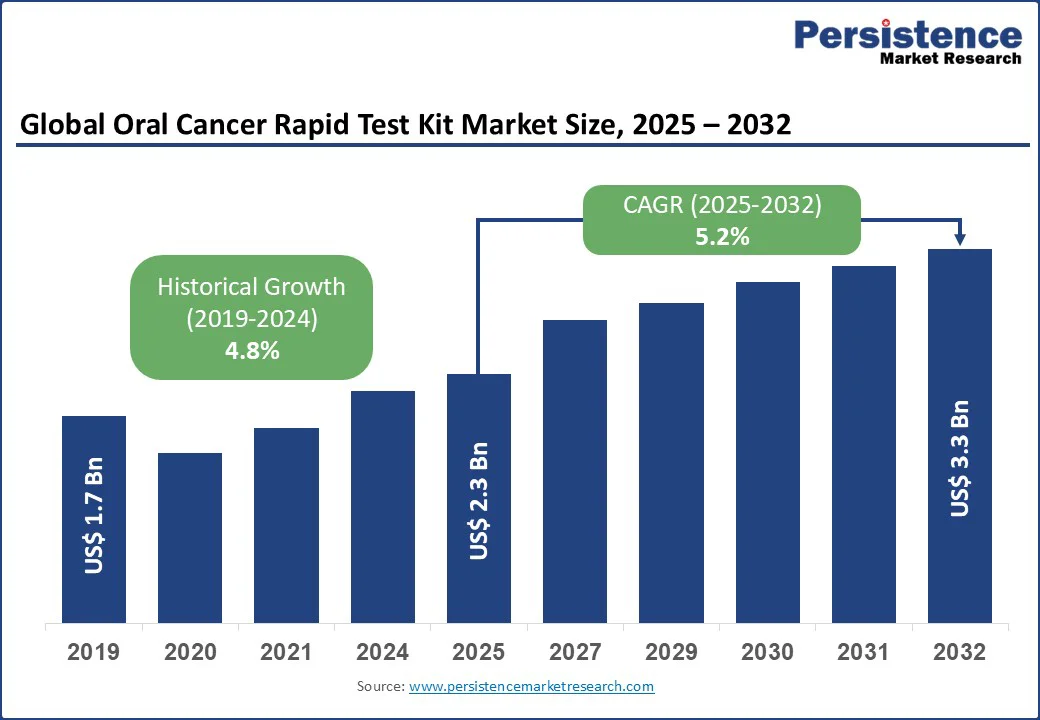
The global oral cancer rapid test kit market size is likely to be valued at US$2.3 Bn in 2025 and is expected to reach US$3.3 Bn by 2032, growing at a CAGR of 5.2% during the forecast period from 2025 to 2032.
The rising prevalence of oral cancer, especially among tobacco users and the aging population, coupled with increasing awareness of early detection benefits. The demand for non-invasive, rapid, and cost-effective diagnostic solutions has surged, supported by technological advancements in biomarker-based testing and AI-assisted diagnostics. Additionally, the expansion of healthcare infrastructure in emerging markets and government initiatives promoting early cancer screening are accelerating adoption, making oral cancer rapid test kits a critical tool for timely diagnosis and improved patient outcomes.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Oral Cancer Rapid Test Kit Market Size (2025E) |
US$2.3 Bn |
|
Market Value Forecast (2032F) |
US$3.3 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
5.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.8% |
Oral cancer remains a major public health concern, particularly in regions with high tobacco and areca nut consumption. In India, over 14 lakh new cancer cases were reported in 2023, with oral cancer being the most prevalent among men, according to government health data. This rising incidence, driven by lifestyle risk factors such as smokeless tobacco and betel quid chewing, has heightened awareness about the importance of early detection, as seen in government programs that have screened millions of individuals for oral cancer. Consequently, there is a growing demand for efficient, non-invasive diagnostic tools, including oral cancer rapid test kits, which can facilitate timely diagnosis and improve treatment outcomes.
To combat this challenge, initiatives such as the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases, and Stroke (NPCDCS), which have established thousands of clinics for early cancer screening, underscore the critical need for rapid, accurate, and accessible diagnostic solutions to manage the increasing burden of oral cancer.
Despite the rising incidence of oral cancer, the adoption of oral cancer rapid test kits remains limited due to insufficient awareness among healthcare professionals and patients. Many clinicians, especially in rural and semi-urban areas, are not fully informed about the availability, advantages, and accuracy of these rapid diagnostic tools. As a result, conventional, time-consuming, and invasive diagnostic methods continue to dominate, delaying timely detection and treatment.
Similarly, patients often lack knowledge about early screening options, leading to late-stage diagnoses and reduced treatment success. The scarcity of targeted training programs, educational campaigns, and outreach initiatives further exacerbates this gap. Enhancing awareness through professional education and public health communication is essential to improve early detection rates and encourage wider adoption of oral cancer rapid test kits, ultimately supporting better patient outcomes and market growth.
The oral cancer rapid test kit market is poised to benefit from significant advancements in artificial intelligence (AI) and home-based testing solutions. AI-powered diagnostic tools can enhance the accuracy and speed of oral cancer detection by analyzing images and biomarker data with high precision. Integrating AI into rapid test kits allows healthcare professionals to identify early-stage oral cancer more efficiently, reducing the reliance on conventional, time-consuming diagnostic procedures.
Simultaneously, the growing trend of home-based testing offers convenience and accessibility, particularly for populations in remote or underserved areas. Patients can perform preliminary screenings in the comfort of their homes, enabling earlier detection and prompt medical consultation. The combination of AI-driven analytics and user-friendly home-based kits presents a significant growth opportunity, encouraging widespread adoption and supporting proactive oral healthcare. This technological innovation is expected to drive market expansion and improve overall patient outcomes.
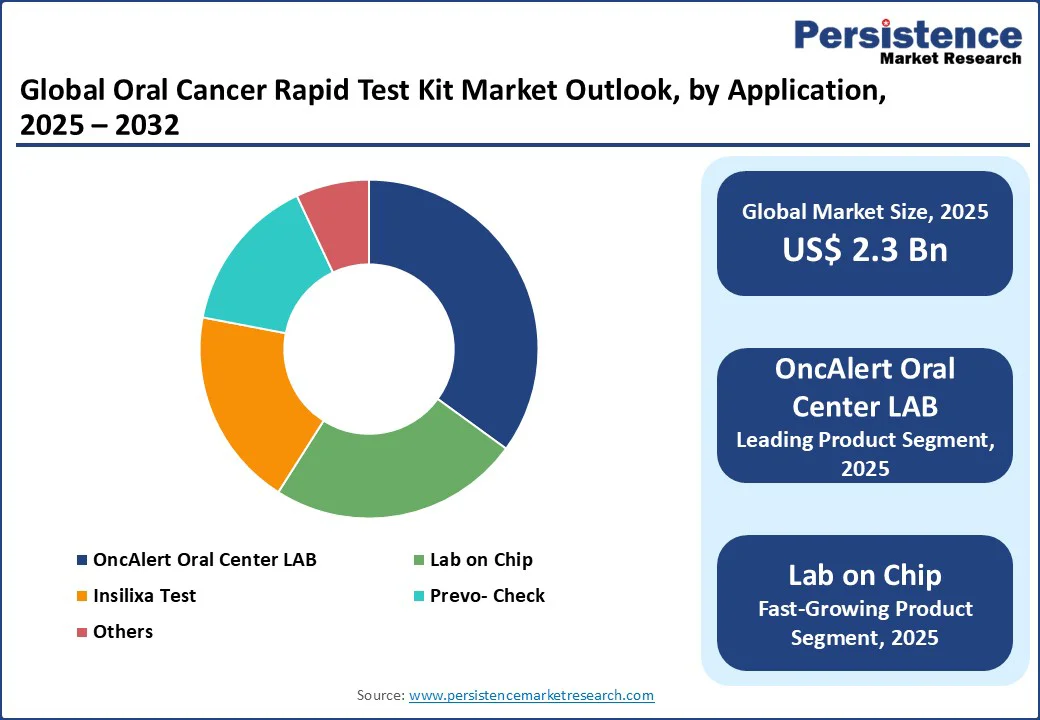
OncAlert Oral Cancer LAB dominates the oral cancer rapid test kit market, holding a 35% market share in 2025, driven by its innovative non-invasive saliva-based testing. Approved in Europe with a CE mark, the test demonstrates approximately 90% sensitivity and is widely adopted in hospitals and diagnostic centers for its ease of use and affordability. In 2024, around half of U.S. diagnostic centers implemented OncAlert for routine oral cancer screening, reinforcing its position as the leading solution. Its reliability and user-friendly design have made it a preferred choice for healthcare professionals aiming to improve early detection rates.
The Lab on Chip is the fastest-growing product segment, fueled by advancements in biosensor technology and device miniaturization. Its portability and rapid results have led to steadily rising adoption in diagnostic centers, offering an efficient, on-the-spot oral cancer screening solution.
Immunoassay leads the oral cancer rapid test kit market, holding a 40% market share in 2025, driven by its high sensitivity and specificity in detecting cancer biomarkers. In 2024, approximately 60% of rapid test kits utilized immunoassay principles, particularly favored in hospital settings for their reliability and consistent performance. The accuracy and ease of integration into existing clinical workflows have made immunoassay the preferred choice among healthcare providers, supporting early and precise oral cancer detection.
Sensor-based test kits represent the fastest-growing principle type, propelled by AI integration and expanding point-of-care applications. The adoption of sensor-based systems is increasing in both home-based and clinical diagnostics, offering rapid, on-site results. Advances in biosensor technology and miniaturization are driving this growth, making sensor-based kits an attractive solution for early oral cancer screening and fostering broader market expansion.
Hospitals dominate the oral cancer rapid test kit market, holding a 45% market share in 2025, driven by high diagnostic volumes and advanced healthcare infrastructure. In 2024, approximately 70% of oral cancer screenings in the U.S. were conducted in hospitals, benefiting from integrated diagnostic workflows and experienced clinical staff. The combination of reliable testing protocols and centralized facilities has made hospitals the preferred end-use setting for early detection and routine screening of oral cancer, supporting consistent patient outcomes.
The consumer segment is the fastest-growing end-use category, fueled by the increasing popularity of home-based testing kits. Adoption among consumers rose by 15% in 2024, driven by convenient and user-friendly products such as QuickBlue and Cancinstancet™. These kits enable preliminary screening in home settings, promoting early detection and timely medical consultation, and are expected to significantly expand by offering accessible solutions outside traditional healthcare facilities.
Hospitals lead as the primary application for oral cancer rapid test kits, holding a 45% market share in 2025, due to their central role in routine screening and diagnosis. In 2024, around 60% of oral cancer diagnoses globally were conducted in hospitals, driven by high patient volumes, access to specialists, and well-established clinical workflows. The presence of trained medical staff and integrated diagnostic infrastructure makes hospitals the preferred setting for accurate and timely detection of oral cancer, reinforcing their dominance.
Diagnostic centers are the fastest-growing application segment, propelled by the adoption of advanced diagnostic technologies. Usage of oral cancer rapid test kits in these centers increased by approximately 10% in 2024, particularly in urban areas, due to faster results and specialized testing capabilities. The growth of diagnostic centers highlights a shift toward decentralized, technology-driven screening solutions that complement traditional hospital-based testing.
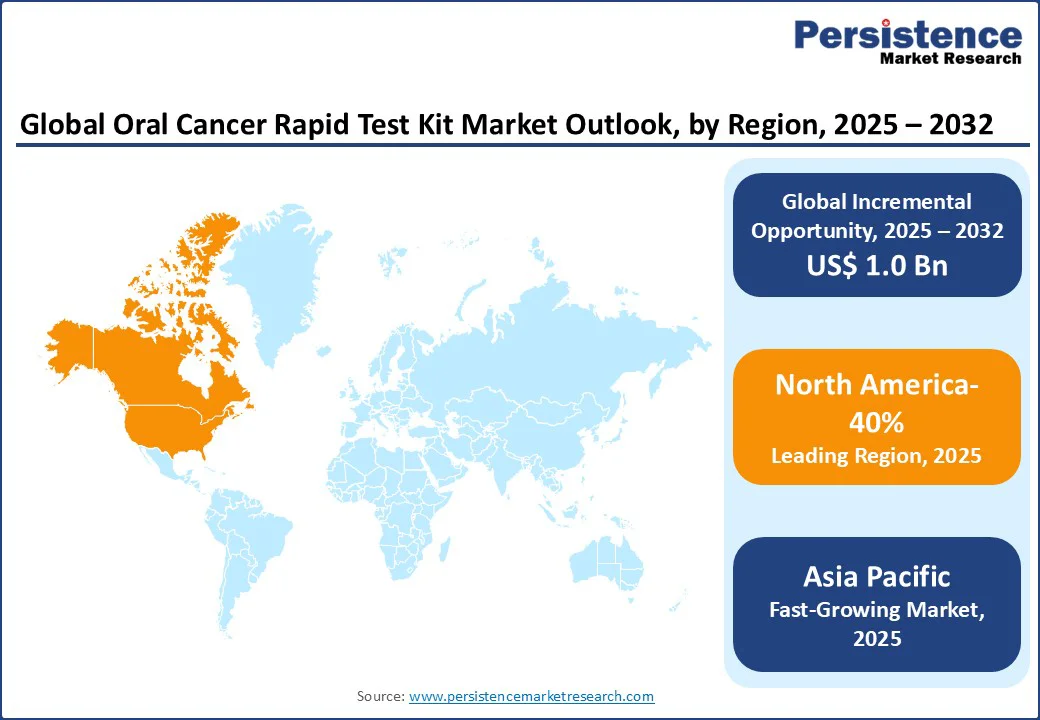
North America holds the largest share of the oral cancer rapid test kit market, accounting for 40% in 2025, driven by advanced healthcare infrastructure and high awareness of early detection. The United States leads the region, with over 54,500 new oral cancer cases reported in 2022 and widespread insurance coverage supporting diagnostic testing. High adoption rates in hospitals and diagnostic centers further reinforce market growth. In Canada, public health campaigns and government funding have encouraged adoption, with approximately 15% of dental clinics utilizing rapid test kits. Overall, strong healthcare systems and awareness initiatives continue to drive the North American market.
Europe holds a notable share of the oral cancer rapid test kit market, driven by rising awareness of early detection and strong regulatory support for diagnostic innovations. Countries such as Germany, France, and the UK are witnessing increased adoption in hospitals and diagnostic centers due to advanced healthcare infrastructure and well-established preventive care programs. Government initiatives and public health campaigns promoting oral cancer screening have further encouraged uptake among patients. Additionally, the region benefits from approvals of CE-marked rapid test kits, ensuring high-quality and reliable diagnostics, which continue to support steady market growth across Europe.
Asia Pacific is the fastest-growing market for oral cancer rapid test kits, driven by increasing oral cancer prevalence, rising healthcare awareness, and expanding healthcare infrastructure. Countries such as India, China, and Japan are witnessing significant adoption in hospitals and diagnostic centers, supported by government initiatives and screening programs. Rapid urbanization and the growing availability of affordable, user-friendly test kits have further accelerated market growth. Additionally, the rising popularity of home-based testing solutions in the region is boosting early detection rates, positioning Asia Pacific as a key growth market for oral cancer rapid diagnostic tools over the coming years.
The global oral cancer rapid test kit market is highly competitive, characterized by continuous innovation and technological advancements. Companies are focusing on developing non-invasive, accurate, and cost-effective diagnostic solutions to strengthen their market position. Strategies such as product launches, strategic collaborations, and expansion into emerging markets are driving growth. Increasing emphasis on research and development, coupled with the rising demand for early detection tools, is intensifying competition. Market participants are also investing in marketing and distribution networks to enhance accessibility and adoption globally.
The oral cancer rapid test kit market is projected to reach US$ 2.3 Bn in 2025, driven by rising oral cancer cases and early detection demand.
Key drivers include increasing oral cancer incidence, technological advancements, and growing awareness of early screening.
The oral cancer rapid test kit market will grow from US$2.3 Bn in 2025 to US$3.3 Bn by 2032, with a CAGR of 5.2%.
Opportunities include AI-driven diagnostics, home-based testing, and expansion in emerging markets such as India and China.
Leading players include Thermo Fisher Scientific Inc., Vigilant Biosciences, Viome Life Sciences, F. Hoffmann-La Roche AG, and Quest Diagnostics Incorporated.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Principle Type
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author