ID: PMRREP33711| 200 Pages | 13 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

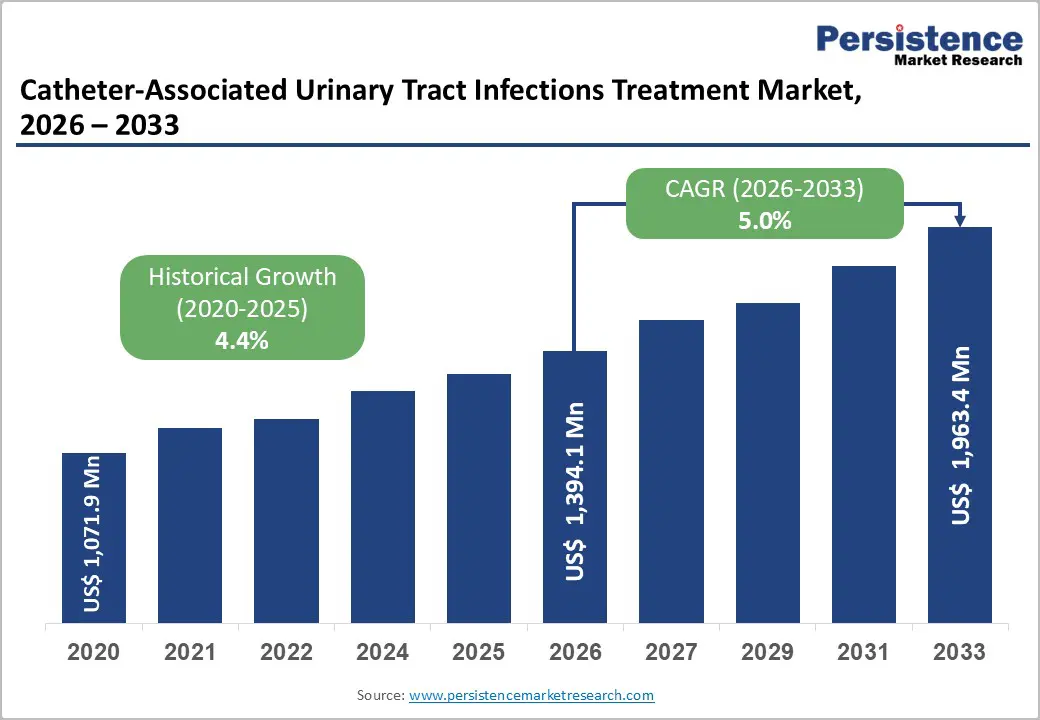
The global catheter-associated urinary tract infections treatment market size is likely to be valued at US$1,394.1 million in 2026 and projected to reach US$1,963.4 million by 2033 growing at a CAGR of 5.0% during the forecast period from 2026 to 2033.
The market growth is driven by rising hospital admissions, increasing use of indwelling catheters, and growing awareness of hospital-acquired infections. Treatment mainly relies on targeted antibiotic therapies, supported by improved diagnostic practices and infection-control protocols. Hospitals remain the primary treatment centers because of higher catheter use in critical and post-surgical care. Growing antimicrobial resistance is encouraging demand for advanced therapies and better catheter management, shaping innovation and clinical practices across global healthcare systems.
| Key Insights | Details |
|---|---|
|
Catheter-Associated Urinary Tract Infections Treatment Market Size (2026E) |
US$1,394.1 Mn |
|
Market Value Forecast (2033F) |
US$1,963.4 Mn |
|
Projected Growth (CAGR 2026 to 2033) |
5.0% |
|
Historical Market Growth (CAGR 2020 to 2024) |
4.4% |
Prolonged catheter dwell time is one of the most critical drivers influencing the catheter-associated urinary tract infection (CAUTI) treatment market. When urinary catheters remain in place for extended periods, they create a direct pathway for bacteria to enter the urinary tract, significantly increasing the likelihood of infection. Over time, biofilms begin to form on catheter surfaces, making bacterial colonies more resistant to host defenses and antibiotic treatment. This is particularly common in immobilized patients, individuals with neurological disorders, and those recovering from major surgeries who require continuous urinary drainage. As catheter duration increases, so does the complexity and severity of infections, often necessitating stronger or combination antibiotic therapies. Hospitals managing long-stay patients in intensive care units and long-term wards face a higher burden of CAUTI cases due to unavoidable catheter dependence.
Additionally, delayed catheter removal often results from clinical caution, staff shortages, or lack of monitoring protocols, further extending dwell time. These factors collectively contribute to rising infection rates, increasing diagnostic testing, prolonged hospital stays, and repeated treatment cycles. As a result, healthcare providers are compelled to invest more in CAUTI management, including culture-guided antibiotic therapy and supportive care. The persistent reliance on long-term catheterization continues to sustain steady demand for CAUTI treatment solutions across hospitals, long-term care facilities, and rehabilitation centers, reinforcing its strong impact on overall market growth.
High dependence on culture-based diagnosis acts as a significant restraint in the catheter-associated urinary tract infection (CAUTI) treatment market, primarily due to the time-intensive nature of conventional urine culture testing. In catheterized patients, clinicians often rely on laboratory culture results to identify the causative pathogen and determine antibiotic susceptibility before initiating targeted therapy. However, standard urine cultures typically require 48–72 hours to generate actionable results. During this waiting period, physicians may delay definitive treatment, especially in clinically stable patients, to avoid unnecessary or inappropriate antibiotic use.
This diagnostic lag contributes to a “watchful waiting” approach, particularly in cases where symptoms are mild or non-specific, such as low-grade fever or altered urine appearance. In such scenarios, empirical antibiotic initiation is often avoided to comply with antimicrobial stewardship guidelines. As a result, overall antibiotic utilization for CAUTI treatment may be lower than expected despite confirmed bacterial presence. Additionally, the high prevalence of asymptomatic bacteriuria in catheterized patients further complicates interpretation, increasing clinicians' hesitation to initiate therapy without culture confirmation.
The reliance on culture-based diagnosis also impacts hospital workflow and treatment efficiency. Delayed therapeutic decisions can prolong patient monitoring, increase bed occupancy, and shift focus toward catheter management rather than pharmacological intervention. From a market perspective, this dependency limits the rapid uptake of treatment therapies and reduces demand for broad-spectrum agents. Until faster, more reliable diagnostic alternatives gain widespread adoption, culture-driven delays will continue to constrain timely CAUTI treatment and market growth.
Rapid point-of-care (POC) diagnostics represent a high-value opportunity in the Catheter-Associated Urinary Tract Infections (CAUTI) treatment market by addressing delays in accurate diagnosis and targeted therapy. Traditional laboratory urine cultures often require 24–72 hours to identify pathogens and antibiotic susceptibility, during which patients are treated empirically with broad-spectrum antibiotics. This approach increases the risk of inappropriate therapy, antimicrobial resistance, prolonged hospital stays, and higher treatment costs.
Bedside POC diagnostic tests can rapidly detect CAUTI-causing pathogens and resistance markers within minutes to a few hours, enabling clinicians to initiate precise, culture-guided treatment at an early stage. These technologies may include molecular assays, biosensors, microfluidic platforms, or rapid antigen-based tests designed specifically for catheterized patients. Faster identification supports improved clinical outcomes, reduces unnecessary antibiotic exposure, and aligns with hospital antibiotic stewardship programs.
From a market perspective, growing pressure on hospitals to reduce healthcare-associated infections and meet quality benchmarks is accelerating demand for rapid diagnostics. Adoption is particularly strong in intensive care units, emergency departments, and long-term care facilities where catheter use is high. As healthcare systems prioritize efficiency, cost containment, and resistance management, rapid POC diagnostics are positioned to become an essential component of CAUTI treatment and infection control strategies.
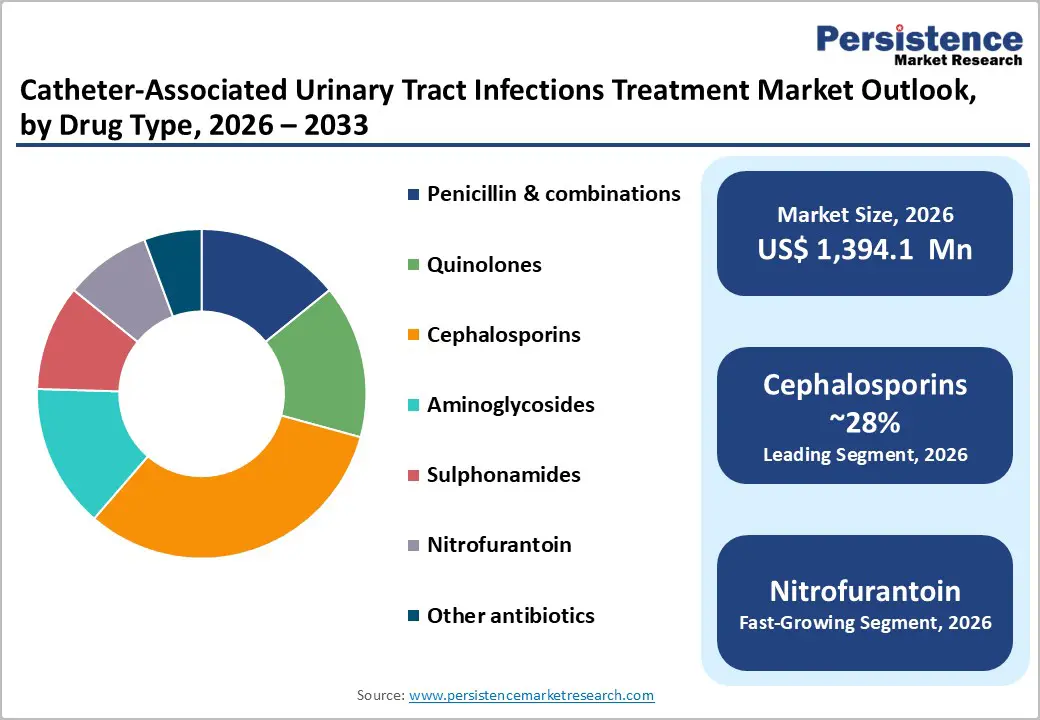
Cephalosporins account for the highest share in the Catheter-Associated Urinary Tract Infections (CAUTI) treatment market due to their broad-spectrum efficacy, clinical versatility, and established safety profile. CAUTI is commonly caused by gram-negative bacteria such as Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis, as well as some gram-positive pathogens. Cephalosporins, especially third- and fourth-generation variants, are highly effective against these uropathogens, making them a preferred choice for empiric therapy when immediate treatment is necessary before culture results are available.
Their dual administration allows for both oral and intravenous administration, providing flexibility in treating both mild and severe infections. Intravenous cephalosporins are often used in hospitalized or critically ill patients with severe CAUTI, whereas oral formulations are suitable for step-down therapy or outpatient management. Compared to other antibiotics like aminoglycosides, which carry higher risks of nephrotoxicity and ototoxicity, cephalosporins offer a safer profile, significant for elderly and catheterized patients who may already have comorbidities.
Additionally, cephalosporins are widely included in hospital antibiotic protocols and stewardship programs due to predictable pharmacokinetics and well-documented efficacy, ensuring adherence to clinical guidelines. This combination of broad activity, safety, and versatility drives their dominant market share in CAUTI treatment globally, reinforcing their position as the first-line therapy in both acute and long-term care settings.
Hospital pharmacies hold the highest share among distribution channels in the Catheter-Associated Urinary Tract Infections (CAUTI) treatment market due to the clinical setting and nature of patient care required. CAUTI predominantly affects hospitalized patients, especially those in intensive care units, post-surgical wards, and long-term care facilities where catheter use is frequent. These patients often require intravenous antibiotics, culture-guided therapy, and continuous monitoring, which are not easily managed through retail or online pharmacies.
Hospital pharmacies ensure timely access to broad-spectrum and targeted antibiotics, including cephalosporins, quinolones, and aminoglycosides, facilitating adherence to standardized treatment protocols. They also play a key role in infection-control measures, such as proper drug storage, dispensing, and stewardship programs, which are critical to preventing antimicrobial resistance and ensuring effective CAUTI management.
Moreover, hospital pharmacies are integrated into the hospital’s clinical workflow, allowing physicians and nurses to coordinate immediate therapy initiation based on laboratory results or rapid diagnostics. Other channels, such as drug stores, retail pharmacies, or online platforms, primarily serve outpatient or follow-up care, where oral antibiotics may be prescribed. However, the volume and criticality of inpatient CAUTI cases make hospital pharmacies the dominant distribution channel, reflecting their central role in effective, timely, and guideline-compliant treatment delivery.
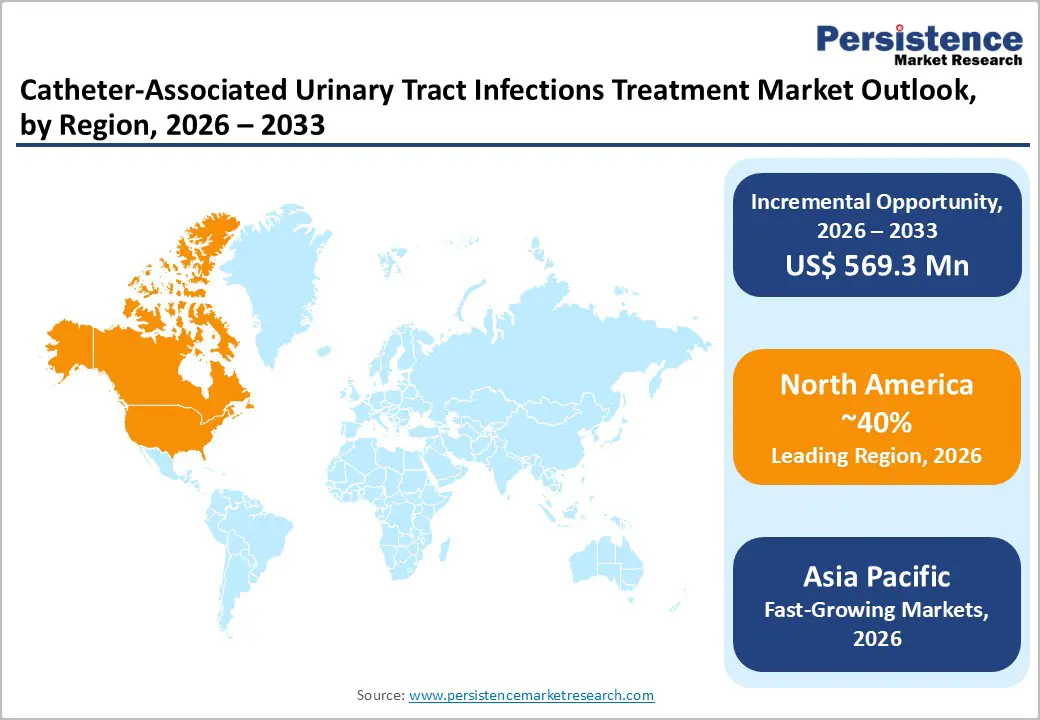
North America remains a leading region in the Catheter-Associated Urinary Tract Infections (CAUTI) treatment market, driven by advanced healthcare infrastructure, high healthcare spending, and widespread adoption of infection prevention and control practices. The United States, in particular, commands a dominant share within the region due to its large patient base, high rates of catheter usage in hospitals and long-term care facilities, and strong emphasis on clinical guideline adherence to manage CAUTI incidences.
Hospitals and healthcare facilities in the U.S. benefit from robust reimbursement frameworks that support comprehensive diagnostic and treatment modalities for infection management, encouraging early intervention and tailored antibiotic therapy. North American trends also show increased use of antimicrobial-coated catheters and preventive strategies aimed at reducing infection rates and associated clinical burdens. In addition, continuous efforts in antimicrobial stewardship and research into enhanced diagnostic tools help optimize antibiotic use and combat resistance, a growing concern in CAUTI treatment. Overall, the region’s combination of technological advances, structured infection control policies, and proactive healthcare practices sustains its leadership in the CAUTI treatment market.
Asia Pacific is emerging as a dynamic growth regional market due to rapid improvements in healthcare infrastructure, particularly in countries like China, India, Japan, and South Korea, expanding access to diagnostic and treatment services in hospitals and outpatient facilities. Rising catheter usage linked to increasing hospital admissions, surgical procedures, and an aging population is driving higher CAUTI incidence, fueling demand for effective antibiotic therapies and infection management solutions. Governments and healthcare providers are emphasizing infection control policies and awareness campaigns that promote early diagnosis and treatment adoption.
Additionally, a growing middle class with higher disposable incomes is increasing healthcare utilization and adoption of advanced therapies. Asia Pacific’s large and diverse patient base, combined with investments in rapid diagnostics and antimicrobial therapies, presents significant market potential. While challenges such as antibiotic resistance and rural healthcare disparities remain, ongoing healthcare reforms and telemedicine expansion are helping bridge gaps, accelerating overall market growth and positioning the region as a key emerging market for CAUTI treatment.
The competitive landscape of the catheter-associated urinary tract infections (CAUTI) treatment market is highly dynamic and innovation-driven, with players focusing on expanding portfolios and enhancing market presence. Companies are investing in R&D, new product development, antimicrobial coatings, and smart catheter technologies to improve patient outcomes and reduce infection rates. Strategic initiatives such as partnerships, collaborations, and geographic expansion are commonly pursued to strengthen footholds in both emerging and established markets. Additionally, smaller innovators and regional manufacturers contribute niche solutions, fostering technological advancement and overall market growth, making the competitive environment highly dynamic and oriented toward continuous innovation.
The global catheter-associated urinary tract infections treatment market is projected to be valued at US$1,394.1 Mn in 2026.
Growing use of indwelling catheters in surgical, ICU, and elderly patients increases infection risk, boosting the need for targeted therapies.
The global market is poised to witness a CAGR of 5.0% between 2026 and 2033.
Expanding adoption of catheters with antimicrobial coatings to prevent infection and reduce hospital-acquired CAUTI incidence.
Pfizer Inc., Merck & Co., Inc., GlaxoSmithKline (GSK), AstraZeneca, and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Drug Type
By Indication
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author