ID: PMRREP34325| 199 Pages | 25 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

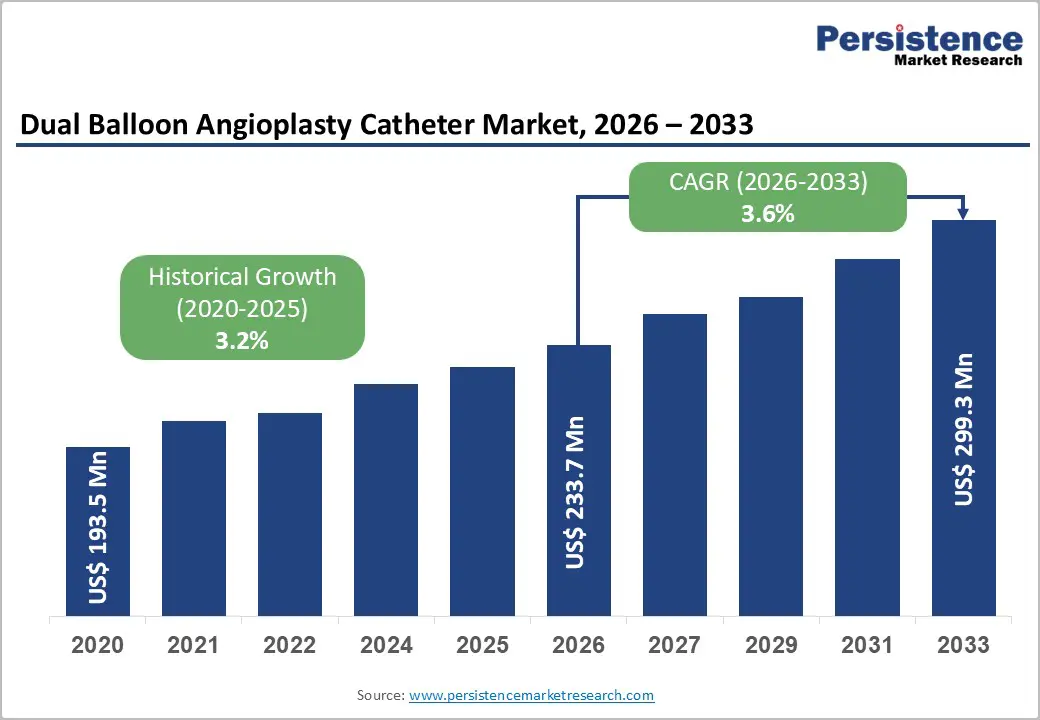
The global dual balloon angioplasty catheter market size is likely to be valued at US$233.7 million in 2026, and is expected to reach US$299.3 million by 2033, growing at a CAGR of 3.6% during the forecast period from 2026 to 2033, driven by the increasing prevalence of complex coronary lesions, rising demand for minimally invasive interventions in peripheral artery disease, and advancements in multi-layer and scoring dual balloon catheter designs.
Growing demand for precise, lesion-specific dual balloon angioplasty catheters, especially multi-layer variants for coronary indications, is accelerating adoption across end-uses. Advances in customized and drug-eluting balloons are further boosting uptake by offering better vessel preparation and reduced restenosis. Increasing recognition of dual balloon angioplasty catheters as critical for calcified lesion treatment and bifurcation stenting in emerging cardiovascular markets remains a major driver of market growth.
| Global Market Attributes | Key Insights |
|---|---|
| Dual Balloon Angioplasty Catheter Market Size (2026E) | US$233.7 Mn |
| Market Value Forecast (2033F) | US$299.3 Mn |
| Projected Growth (CAGR 2026 to 2033) | 3.6% |
| Historical Market Growth (CAGR 2020 to 2025) | 3.2% |
Increasing Demand for Complex Coronary Lesions and Minimally Invasive Interventions
The rising demand for the treatment of complex coronary lesions and minimally invasive interventions is becoming a significant driver in the cardiovascular care landscape. Complex coronary lesions such as chronic total occlusions, bifurcation lesions, calcified arteries, and multivessel disease are increasingly prevalent due to aging populations, longer life expectancy, and the growing incidence of lifestyle-related conditions, including diabetes, obesity, and hypertension. These factors are resulting in a higher proportion of patients presenting with advanced and anatomically challenging coronary artery disease that requires sophisticated, technology-driven treatment approaches. This growing procedural demand is reflected in real-world clinical data; for instance, the National Interventional Council (NIC) registry under the Cardiological Society of India reported that 438,351 percutaneous coronary interventions (PCIs) were performed nationwide in a single year (2018), representing a 13.14% year-on-year increase, underscoring the expanding reliance on catheter-based cardiac treatments.
There is a strong clinical and patient-driven shift toward minimally invasive interventions. Compared with conventional open-heart surgery, minimally invasive procedures such as PCI offer reduced procedural trauma, shorter hospital stays, lower complication risks, and faster recovery timelines. This preference is especially evident among elderly and high-risk patients who may not be suitable candidates for surgical revascularization. Continuous advancements in interventional cardiology technologies, including enhanced guidewires, imaging-guided catheters, next-generation drug-eluting stents, and atherectomy systems, have significantly improved physicians’ ability to treat complex coronary anatomies through minimally invasive techniques, further reinforcing this market trend.
Risk of Procedure-Related Complications
The risk of procedure-related complications represents a key restraint for the dual balloon angioplasty catheter market, particularly in complex coronary and peripheral interventions. While dual balloon systems are designed to improve lesion access, vessel preparation, and procedural precision, their use inherently involves navigating delicate and often diseased vasculature. In cases involving heavily calcified, tortuous, or bifurcated vessels, there is an elevated risk of complications such as vessel dissection, perforation, thrombosis, or distal embolization. The presence of two balloons increases procedural complexity, requiring precise positioning and pressure control to avoid unintended vessel trauma.
Prolonged balloon inflation times, which are sometimes necessary to achieve optimal lesion modification, can temporarily compromise blood flow, increasing the risk of ischemia, arrhythmias, or hemodynamic instability, particularly among elderly or high-risk patient populations. Improper balloon sizing or over-inflation may further contribute to restenosis or adverse vessel remodeling, potentially resulting in repeat interventions. These risks are amplified in clinical settings where operator experience with dual balloon techniques is limited, as procedural success is closely tied to technical expertise.
Regulatory actions also underscore these safety concerns; for instance, the U.S. Food and Drug Administration (FDA) classified the recall of nearly 17,000 intra-aortic balloon catheter kits as a Class I recall after a manufacturing defect causing incomplete inflation was identified, an issue that was linked to 31 reported injuries and three deaths. This example highlights how device-related failures can significantly impact patient safety and reinforce caution in the adoption of balloon-based angioplasty technologies.
Innovations in Drug-Eluting and Customized Balloon Platforms
Innovations in drug-eluting and customized balloon platforms are significantly enhancing the effectiveness of minimally invasive vascular interventions. Drug-eluting balloons (DEBs) are engineered to deliver therapeutic agents directly to the vessel wall during balloon inflation, reducing smooth muscle cell proliferation and lowering the risk of restenosis without leaving a permanent implant behind. Recent advancements have focused on improving drug transfer efficiency through optimized coating technologies, controlled drug release mechanisms, and enhanced balloon surface designs that ensure uniform drug distribution even in complex or irregular lesions.
The development of customized balloon platforms is enabling more precise, patient-specific treatment approaches. Balloons are now available in a wider range of diameters, lengths, compliance levels, and pressure tolerances, allowing clinicians to tailor interventions based on vessel anatomy, lesion morphology, and disease severity. Specialized designs such as scoring, cutting, and dual-balloon configurations further improve plaque modification while minimizing vessel trauma. Integration with advanced imaging techniques also supports accurate sizing and placement, improving procedural predictability.
Product Type Insights
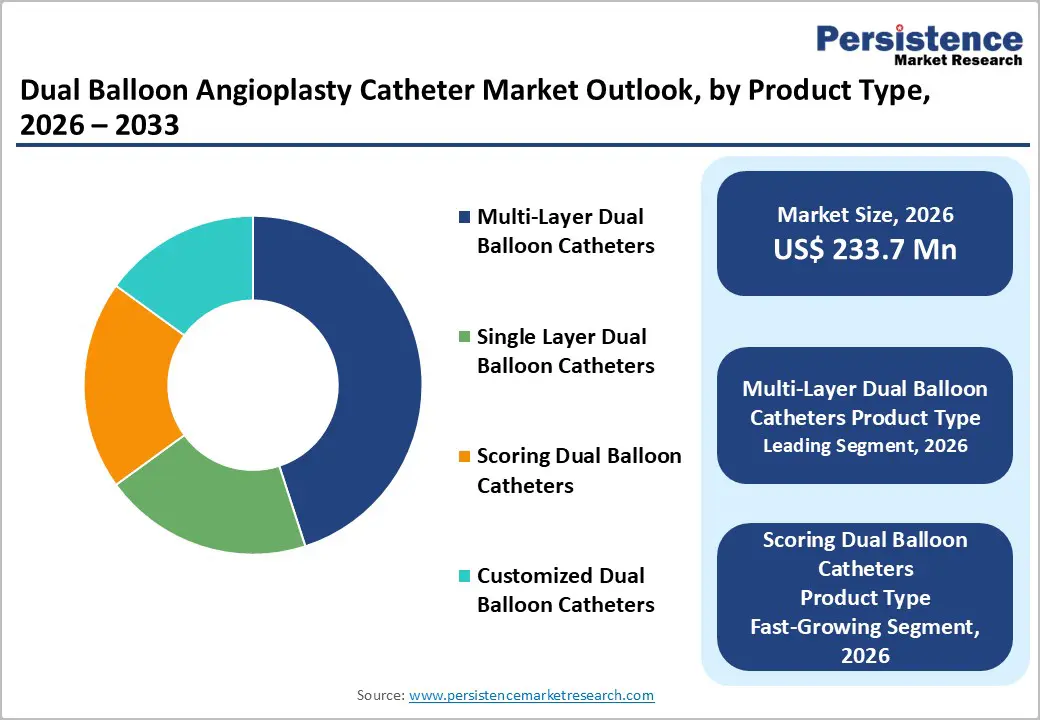
Multi-layer dual balloon catheters are anticipated to dominate the market, accounting for approximately 35% of the market share in 2026, due to their enhanced structural performance and clinical versatility. The multi-layer design improves balloon strength, pressure control, and durability, enabling safer and more effective treatment of complex and calcified lesions. These catheters offer better resistance to rupture and more predictable expansion, which is critical in high-risk interventions.
Their ability to support precise lesion preparation while minimizing vessel trauma makes them increasingly preferred by interventional specialists. Boston Scientific Corporation’s multi-layer balloon designs are used in some of its angioplasty devices. Boston Scientific’s balloon dilatation catheters, such as the NC TREK™ RX and NC TREK™ OTW, incorporate Multi-Layer CrossFlex2™ balloon technology, which enhances flexibility, trackability, and controlled dilation in challenging vascular lesions.
Scoring dual balloon catheters represents the fastest-growing segment, due to their ability to precisely modify vascular lesions while minimizing vessel trauma. These catheters feature specialized scoring elements on the balloon surface that create controlled micro-incisions in plaque, enhancing expansion and improving stent apposition. This design reduces the risk of dissection, elastic recoil, and restenosis compared with conventional balloons, making them particularly effective in calcified, fibrotic, or complex coronary lesions.
Growing clinical adoption is driven by improved procedural outcomes, shorter recovery times, and compatibility with minimally invasive techniques, positioning scoring dual balloon catheters as a preferred choice in advanced interventional cardiology. Scoreflex NC Scoring PTCA Catheter from OrbusNeich Medical Group Holdings Limited. This device is designed for controlled plaque modification during percutaneous transluminal coronary angioplasty (PCI) procedures, especially in calcified, fibrotic, or complex lesions where standard balloons may be less effective. The Scoreflex NC uses an integrated dual wire scoring system that enhances lesion preparation while maintaining precise dilation and reducing vessel trauma.
Indication Insights
Coronary is expected to lead the market, holding approximately 60% of the share in 2026, driven by the high prevalence of coronary artery disease worldwide. The rising incidence of complex coronary lesions, including bifurcations, chronic total occlusions, and calcified arteries, has increased demand for advanced balloon-based interventions. Coronary procedures benefit from dual balloon technology due to improved lesion access, precise expansion control, and reduced risk of vessel trauma.
Boston Scientific Corporation, a major interventional cardiology device company, offers a broad range of coronary balloon catheters specifically designed for percutaneous coronary interventions (PCI), including products such as the NC Quantum Apex™ PTCA Dilatation Catheter and Emerge™ PTCA Dilatation Catheter. These devices are engineered to optimize deliverability and controlled dilation of coronary artery stenoses, helping clinicians treat coronary artery disease effectively and safely.
Peripheral is likely to be the fastest-growing segment, due to the rising incidence of peripheral artery disease (PAD), increased screening, and expanding treatment access in aging populations. PAD affects arteries outside the heart, especially in the legs, and often presents with complex, calcified lesions that benefit from advanced dual balloon technology.
Improvements in catheter flexibility and trackability have made it easier to navigate tortuous peripheral vasculature, encouraging adoption among interventionists. Medtronic, Inc. has expanded its product offerings in peripheral balloon catheters for the treatment of peripheral artery disease (PAD), including devices such as the Amphirion Plus Percutaneous Transluminal Angioplasty (PTA) Catheter and the Amphirion Deep PTA Balloon Catheter, designed specifically for below the knee and below the ankle interventions.
End-user Insights
Hospitals are expected to dominate the market, contributing nearly 70% of revenue in 2026, due to their central role in managing complex cardiovascular procedures. Hospitals are equipped with advanced catheterization laboratories, imaging infrastructure, and multidisciplinary clinical teams required for high-risk angioplasty interventions. They also handle a large volume of emergency and elective cardiovascular cases, including complex coronary and peripheral procedures that require specialized devices.
Saint Luke’s Mid America Heart Institute (MAHI) in Kansas City, Missouri, is a leading hospital-based cardiovascular care center with multiple cardiac catheterization laboratories where advanced interventional procedures, including complex angioplasty and PCI, are routinely performed. The institute’s extensive infrastructure, including dedicated cath labs and multidisciplinary cardiovascular teams, enables high procedural volumes and adoption of advanced catheter-based devices.
Catheterization labs represent the fastest-growing segment, as they serve as the primary settings for advanced angioplasty and minimally invasive vascular procedures. As the prevalence of cardiovascular diseases rises, more hospitals and specialty clinics are investing in dedicated cath labs to expand procedural capacity.
These labs are increasingly equipped with state-of-the-art imaging, navigation, and interventional tools that support complex diagnostics and therapeutic interventions, including dual balloon angioplasty. Norton Clark Hospital has opened a newly renovated cardiac catheterization (cath) lab and unveiled a new CT scanner with cardiovascular capabilities. The upgrades are the result of a five-month construction process and a more than US$7 million investment. They also mean the hospital now offers heart care for patients using state-of-the-art equipment that produces less radiation and better images.
North America Dual Balloon Angioplasty Catheter Market Trends
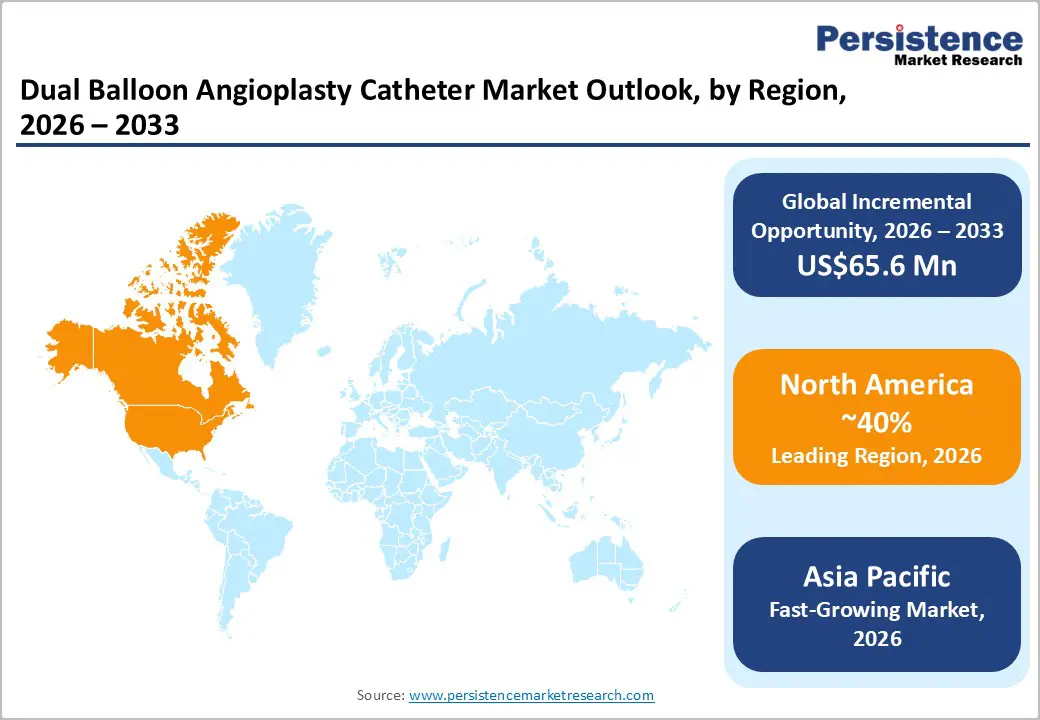
North America is projected to dominate the market, capturing nearly 40% of the global dual balloon angioplasty catheter market share in 2026, driven by the region’s advanced cardiac care infrastructure, strong research and development capabilities, and high public awareness of minimally invasive benefits. Procedural systems in the U.S. and Canada provide extensive support for intervention programs, ensuring wide accessibility of dual balloon angioplasty catheters across coronary, peripheral, and complex populations. Increasing demand for scoring, convenient, and easy-to-navigate forms is further accelerating adoption, as these formats improve outcomes and reduce barriers associated with standard balloons.
Innovation in dual balloon angioplasty catheter technology, including stable multi-layer, improved drug-eluting delivery, and targeted lesion enhancement, is attracting significant investment from both public and private sectors. Government initiatives and AHA campaigns continue to promote use against restenosis risks, procedural complexity, and emerging PAD threats, creating sustained market demand. The growing focus on peripheral grades and specialty uses, particularly for hospitals and others, is expanding the target applications for dual balloon angioplasty catheters.
Europe Dual Balloon Angioplasty Catheter Market Trends
Market growth in Europe is fueled by rising awareness of the benefits of minimally invasive procedures, robust healthcare infrastructures, and government-supported cardiac initiatives. Countries such as Germany, France, and the U.K. have well-established interventional cardiology frameworks that facilitate the routine use of dual balloon angioplasty catheters and encourage the adoption of innovative delivery technologies. These advanced catheter designs are particularly well-suited for coronary patients, regulation-conscious clinicians, and peripheral interventions, enhancing vessel patency and procedural success.
Technological advancements in dual balloon angioplasty catheter development, such as enhanced scoring, application-targeted delivery, and improved customized grades, are further boosting market potential. European authorities are increasingly supporting research and trials for catheters against both routine and specialized needs, strengthening market confidence. The growing emphasis on convenient, low-restenosis options is aligned with the region’s focus on preventive cardiology and reducing reinterventions. Public awareness campaigns and promotion drives are expanding reach in both urban and rural areas, while suppliers are investing in coatings and novel variants to increase efficacy.
Asia Pacific Dual Balloon Angioplasty Catheter Market Trends
Asia Pacific is likely to be the fastest-growing market for dual balloon angioplasty catheters in 2026, driven by rising cardiovascular awareness, increasing government initiatives, and expanding application programs across the region. Countries such as India, China, Japan, and Southeast Asian nations are actively promoting catheter campaigns to address PAD growth and emerging coronary needs. Dual balloon angioplasty catheters are particularly attractive in these regions due to their scalable administration, ease of use, and suitability for large-scale intervention drives in both urban and rural populations.
Technological advancements are supporting the development of stable, effective, and easy-to-deploy dual balloon angioplasty catheters, which can withstand challenging procedural conditions and minimize complication dependence. These innovations are critical for reaching remote facilities and improving overall treatment coverage. Growing demand for coronary, peripheral, and PCI applications is contributing to market expansion. Public-private partnerships, increased cardiac expenditure, and rising investment in catheter research and manufacturing capacity are further accelerating growth. The convenience of catheter delivery, combined with improved outcomes and reduced risk of failure, positions dual balloon angioplasty catheters as a preferred choice.
The global dual balloon angioplasty catheter market features competition between established medtech leaders and emerging vascular suppliers. In North America and Europe, Spectranetics and Eucatech AG lead through strong R&D, distribution networks, and clinical ties, bolstered by innovative multi-layer and scoring programs. In Asia Pacific, Natec Medical advances with localized solutions, enhancing accessibility. Drug-eluting delivery boosts efficacy, cuts restenosis risks, and enables mass integrations across regions. Strategic partnerships, collaborations, and acquisitions merge expertise, expand portfolios, and speed commercialization. Customized formulations solve lesion issues, aiding penetration in complex areas.
Key Industry Developments
The global dual balloon angioplasty catheter market is projected to reach US$233.7 million in 2026.
The rising prevalence of complex coronary lesions and demand for minimally invasive interventions are key drivers.
The dual balloon angioplasty catheter market is poised to witness a CAGR of 3.6% from 2026 to 2033.
Advancements in drug-eluting and customized balloon platforms are key opportunities.
Spectranetics, Eucatech AG, Balton Sp. z o.o., PanMed, and Natec Medical are the key players.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020–2025 |
| Forecast Period | 2026–2033 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Indication
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author