ID: PMRREP28720| 195 Pages | 14 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

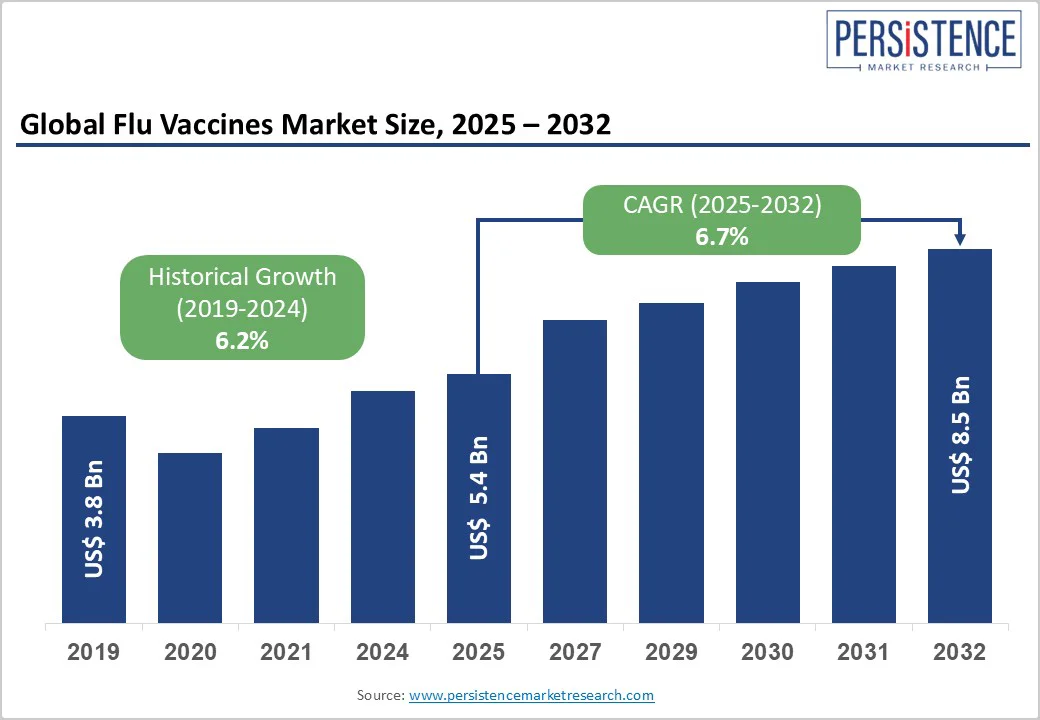
The global flu vaccines market size is likely to value US$ 5.4 billion in 2025 and projected to reach US$ 8.5 billion at a CAGR of 6.7% during the forecast period from 2025 to 2032. Flu Vaccines are biological agents that cause the body to produce antibodies that protect against the influenza virus. Within two weeks of the vaccine, the body begins to produce antibodies. Flu vaccines are becoming increasingly popular because they prevent and minimize the spread of the influenza virus. Due to the expansion of the new target demographic across developing and underdeveloped countries, the market is expanding significantly.
| Global Market Attribute | Key Insights |
|---|---|
| Flu Vaccines Market Size (2025E) | US$ 5.4 Bn |
| Market Value Forecast (2032F) | US$ 8.5 Bn |
| Projected Growth (CAGR 2025 to 2032) | 6.7% |
| Historical Market Growth (CAGR 2019 to 2024) | 6.2% |
The global flu vaccines market is primarily driven by the steady need for large-scale vaccine production and ongoing efforts to modernize manufacturing technologies. According to a recent WHO survey, global seasonal influenza vaccine production capacity has remained stable at approximately 1.53 billion doses, with a heavy dependence on egg-based manufacturing methods. This reliance highlights the growing demand for diversified vaccine production platforms such as cell-based and mRNA technologies to improve efficiency, scalability, and responsiveness to emerging influenza strains.
The continuous risk of seasonal flu outbreaks and periodic pandemics further compels governments and healthcare organizations to strengthen vaccination programs. Increased public health awareness, rising immunization coverage, and support from global health agencies are also boosting vaccine demand. Moreover, advancements in genetic sequencing and strain identification enable the timely development of updated vaccines each flu season. Together, these factors combined with technological upgrades and expanding global immunization initiatives are key drivers accelerating the growth and innovation of the global influenza vaccines market.
The unpredictable nature of seasonal influenza continues to create a persistent gap between the supply and demand of flu vaccines, which poses a challenge to market stability. The severity and spread of influenza vary each year, making it difficult to forecast vaccine requirements accurately. Although global awareness campaigns by health organizations have successfully increased vaccination intent, unexpected fluctuations in infection rates often lead to either vaccine shortages or excess stock.
The World Health Organization’s (WHO) strain predictions and production estimates sometimes differ from actual demand, contributing to market imbalance. Additionally, many manufacturers follow a cautious production strategy to avoid losses from unsold doses, resulting in limited vaccine availability during peak outbreaks or pandemics. This mismatch between production capacity and real-world demand restricts timely access to vaccines, particularly in developing regions, thereby hampering the overall growth of the global flu vaccines market despite growing awareness and preventive healthcare initiatives.
Increasing global awareness about the importance of influenza vaccination is opening significant growth opportunities for the flu vaccines market. Public health campaigns and education programs led by organizations such as the CDC, WHO, and GAVI have boosted vaccine uptake and reduced flu-related hospitalizations and deaths. According to the U.S. Centers for Disease Control and Prevention (CDC), flu vaccinations during the 2019–2020 season prevented approximately 105,000 hospitalizations and 6,300 deaths in the United States alone. Governments across Europe, North America, and the Asia Pacific are further integrating flu vaccination into national immunization schedules, enhancing accessibility and affordability.
In parallel, vaccine manufacturers are strategically expanding their regional presence through product launches, acquisitions, and collaborations. For instance, GSK began shipping its 2025–26 trivalent flu vaccine doses following FDA approval, while Zydus launched India’s first WHO-recommended quadrivalent flu vaccine for the 2025 southern hemisphere. Such initiatives, combined with stronger healthcare infrastructure and awareness campaigns, are expected to create lucrative revenue opportunities for the global flu vaccines market throughout the forecast period.
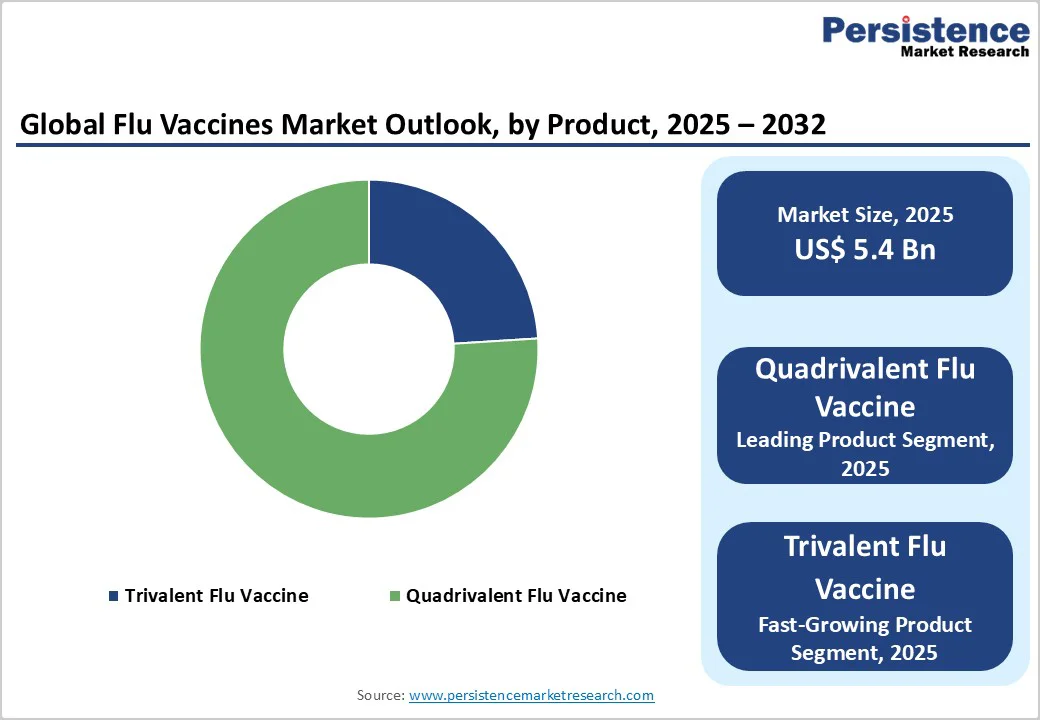
In 2024, the quadrivalent flu vaccine accounted for approximately 74% of the total influenza vaccine market share, driven by its superior effectiveness, affordability, and wide availability across hospitals and clinics. Unlike trivalent formulations, the quadrivalent vaccine provides broader protection by targeting four influenza virus strains, two influenza A and two influenza B types, offering enhanced immunity during flu seasons with unpredictable viral variations. Its comprehensive coverage reduces the risk of mismatch between circulating and vaccine strains, making it the preferred choice for immunization programs worldwide.
Additionally, ongoing approvals and launches of new quadrivalent vaccines by major pharmaceutical companies have further strengthened this segment’s dominance. The growing demand for efficient and accessible flu prevention, supported by government vaccination drives and public health initiatives, continues to fuel the market’s expansion. The combination of clinical effectiveness, convenience, and regulatory support positions the quadrivalent flu vaccine as the leading product in the global influenza vaccine market.
In 2024, intramuscular injections dominated the global flu vaccines market, holding nearly 86% of the total share. This method is preferred due to its proven effectiveness, safety, and rapid absorption. Injecting the vaccine deep into the muscle allows for quick entry into the bloodstream, ensuring a strong and lasting immune response. It is also ideal when intravenous access is difficult or when oral medications are ineffective due to digestive degradation. Healthcare professionals widely favor this route for its accuracy and minimal side effects.
Among alternative dosage forms, nasal sprays emerge as the fastest-growing segment, driven by their needle-free, painless administration and convenience, particularly among children and individuals with needle anxiety. The intradermal shot segment is also gaining traction due to its ability to elicit strong immune responses with lower antigen doses. However, its use remains limited compared to intramuscular and nasal forms due to cost and technical challenges.
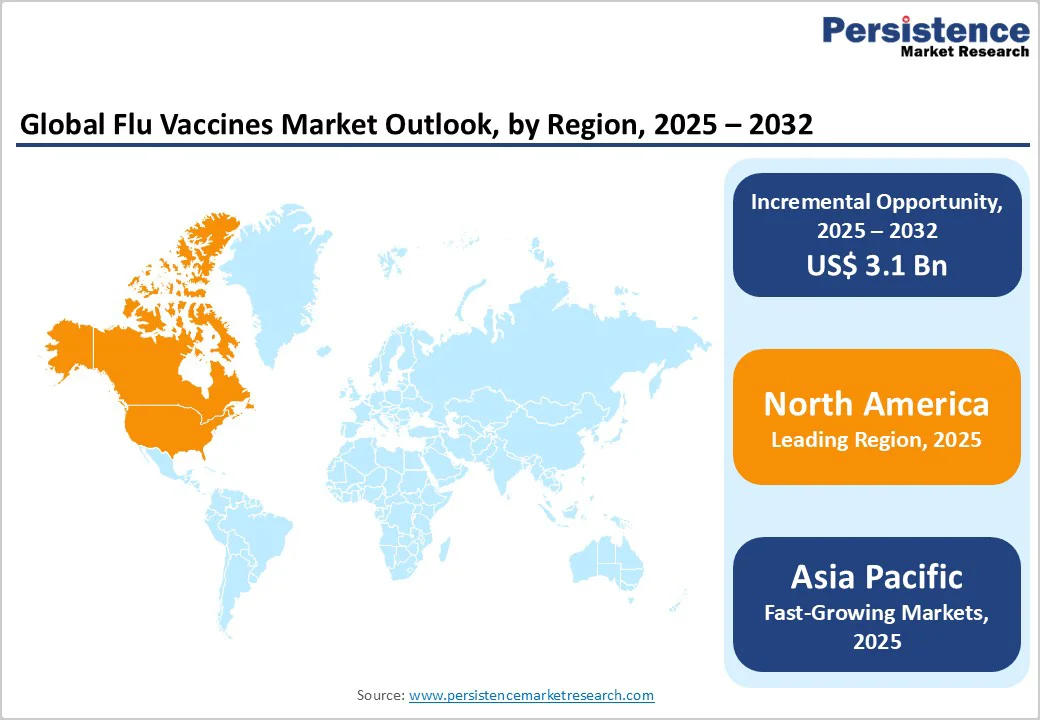
The North American flu vaccines market continues to expand steadily, supported by a growing supply of vaccines from leading manufacturers. Companies such as GSK, Sanofi, and AstraZeneca have significantly increased production capacity to meet rising vaccination demand across the U.S. and Canada. Early distribution and strong government-backed immunization programs have ensured widespread vaccine accessibility through pharmacies, clinics, and hospitals.
Public awareness campaigns by the Centers for Disease Control and Prevention (CDC) and other health agencies are further promoting annual flu vaccination as an essential preventive measure. Additionally, the presence of advanced research facilities and robust healthcare infrastructure supports ongoing development of improved vaccine formulations, including high-dose and adjuvanted variants tailored for elderly populations. Favorable reimbursement policies and the availability of quadrivalent vaccines have also strengthened market adoption. Together, these factors are driving consistent growth and making North America one of the most mature and well-established markets for influenza vaccination globally.
The Asia Pacific flu vaccines market is witnessing strong growth, driven by the rapid development of China’s vaccine manufacturing industry. Chinese biopharmaceutical firms are expanding production capacity and enhancing research capabilities to meet the growing regional demand for seasonal influenza vaccines. Government initiatives promoting self-sufficiency in vaccine production and regulatory support for domestic innovation are accelerating industry advancement.
Collaborations between local vaccine developers and global pharmaceutical companies are also helping to introduce advanced formulations and strengthen supply chains. Public health campaigns in countries such as China, Japan, India, and Australia are increasing vaccination coverage and awareness, particularly among vulnerable populations. The expansion of cold chain infrastructure and the rise of private healthcare facilities are improving vaccine accessibility in urban and semi-urban regions. With ongoing investments in R&D and manufacturing technology, China’s progress is significantly contributing to the overall growth and competitiveness of the Asia Pacific flu vaccines market.
The global flu vaccines market is highly competitive, with major players such as Sanofi, GSK, AstraZeneca, CSL Seqirus, and Moderna leading the industry. These companies focus on expanding production capacity, enhancing vaccine efficacy, and developing next-generation formulations such as cell-based and recombinant vaccines. Strategic collaborations, mergers, and public health partnerships strengthen their market presence. Regional manufacturers, especially in Asia Pacific, are also increasing competition through cost-effective vaccine production and government-backed initiatives. Continuous R&D investments, global immunization programs, and rising demand for quadrivalent vaccines further intensify the competitive dynamics within the flu vaccines market.
Key Industry Developments:
The global flu vaccines market is projected to be valued at US$ 5.4 Bn in 2025.
Rising influenza cases, government vaccination programs, and growing awareness about preventive healthcare drive demand.
The global market is poised to witness a CAGR of 6.7% between 2025 and 2032.
New vaccine technologies, universal flu vaccine research, and expanding immunization coverage in developing regions offer growth.
Companies such Sanofi Pasteur, AstraZeneca, Csl Ltd., Abbott, and GlaxoSmithKline Plc. are some of the major players operating in the global Flu Vaccines Market.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 – 2024 |
| Forecast Period | 2025 – 2032 |
| Market Analysis | Value: US$ Bn and Volume (if Available) |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By Dosage Form
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author