ID: PMRREP20608| 187 Pages | 2 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

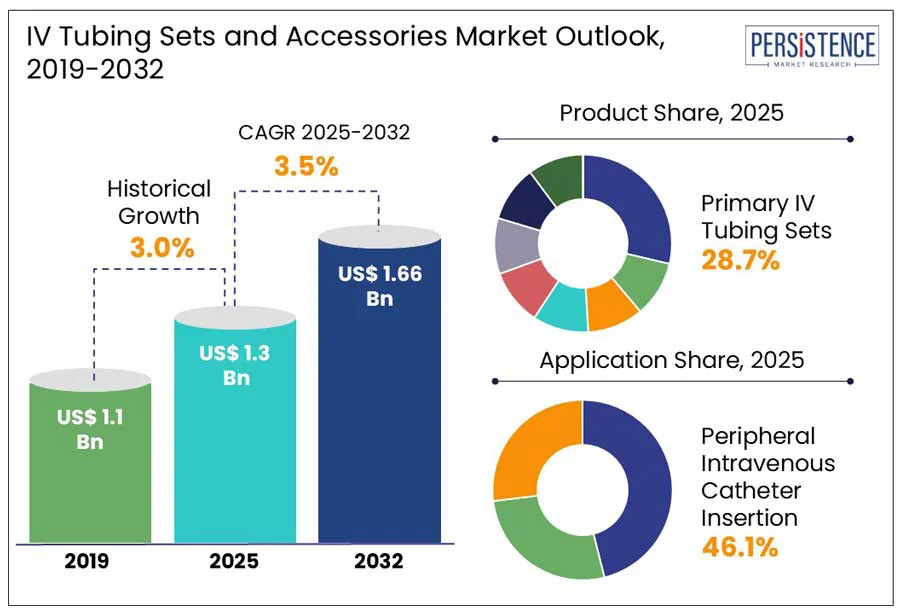
According to the Persistence Market Research report, the global IV tubing sets and accessories market size is expected to reach US$ 1.3 billion by 2025 and is expected to reach US$ 1.7 Bn at a CAGR of 3.5% by 2032. The increasing need for intravenous therapy in chronic illness management and emergency care is driving the global market for IV tubing sets and accessories. Products such as primary and secondary IV sets, extension tubing, vented and non-vented lines, and connectors are widely used in healthcare and homecare settings for administering fluids and medications.
Additionally, the increasing number of outpatient procedures and surgeries is further boosting the demand for IV products in post-surgery recovery and ongoing treatment. Ongoing innovation in materials and manufacturing processes, improved product safety, and user-friendly designs contribute to the growing adoption of IV products globally.
Key Highlights:
|
Global Market Attribute |
Key Insights |
|
IV Tubing Sets and Accessories Market Size (2025E) |
US$ 1.3 Bn |
|
Market Value Forecast (2032F) |
US$ 1.7 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
3.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.0% |
Major sectors have witnessed a shift in recent years from North America and Europe to Asia Pacific in terms of investment in research and development (R&D) and technology, particularly in the life sciences and biotechnology industries. The opportunity for outsourcing biotech projects is boosted by the presence of a skilled workforce and qualified personnel in economies such as India at affordable pricing. India contributed nearly 40% of all investments made in the international engineering and R&D sector in 2016.
Additionally, several governments in these areas have supported the expansion of local biotechnology and healthcare industries by providing tax exemptions for R&D, increasing budgets and funding, forming public and private conglomerates, and attracting foreign direct investments, among other measures.
Over the coming years, a steady increase in R&D spending on medical equipment is anticipated, along with investment in the production of IV tube sets and associated components. By actively expanding their range of IV administration products to meet market demand, these companies are expected to achieve stable and sustained returns.
Stringent regulatory requirements for novel products are a major factor hindering the growth of the IV tubing sets and accessories market globally. The FDA has strict regulatory requirements demanding extensive testing, documentation, and compliance, leading to longer development timelines and increased costs. Manufacturing advanced tubing systems with safety features further adds to production expenses, limiting affordability, especially in low-resource settings.
The introduction of new products is challenging for both small and large device makers owing to the lack of better predictability and openness from the authorities. In addition, concerns such as the risk associated with IV therapy, and healthcare-associated infections (HAIs), necessitate continuous innovation and training to ensure safety. Furthermore, intense competition from domestic manufacturers often results in pricing pressures and reduced profit margins.
Moreover, with the rise in consideration of sustainable manufacturing and disposal of medical equipment, the demand further experiences a decline. These factors are thus set to restrain the growth of the overall market over the projected time frame.
The primary IV tubing sets are projected to account for 28.7% of the IV tubing sets and accessories market in 2025. A thin, bendable plastic tube known as the infusion set is referred to as the primary administration set.
Primary IV tubing sets are widely used in various clinical settings for intermittent or continuous administration of fluids, medications, and nutrients to patients. Their versatility makes them an essential component in routine intravenous therapy. Additionally, they are highly reliable and cost-effective, which further solidifies their dominance compared to secondary sets, extension sets, or more specialized options.
Other tubing sets and accessories are typically used for more specialized procedures or specific patient needs, such as secondary IV tubing for piggyback infusions, extension tubing sets to provide flexibility, and filtered tubing to prevent contamination and ensure safety. Overall, the broad applicability, reliability, and cost-effectiveness of primary IV tubing sets make them the dominant segment in the global market.
Peripheral intravenous catheter insertion is expected to be the dominating segment with 46.1% global IV tubing sets and accessories market value share in 2025. The peripheral IV therapies are minimally invasive and quicker to administer. They are widely used in short-term IV therapies such as fluid administration, medications, and blood draws, in both outpatient and inpatient settings, due to ease of insertion and lower cost compared to more specialized methods. Central venous catheter (CVC) placement and peripherally inserted central catheter (PICC) line insertion, on the other hand, are long-term treatments used for more complex cases, which limits their regular use in comparison to peripheral IVs line insertions.
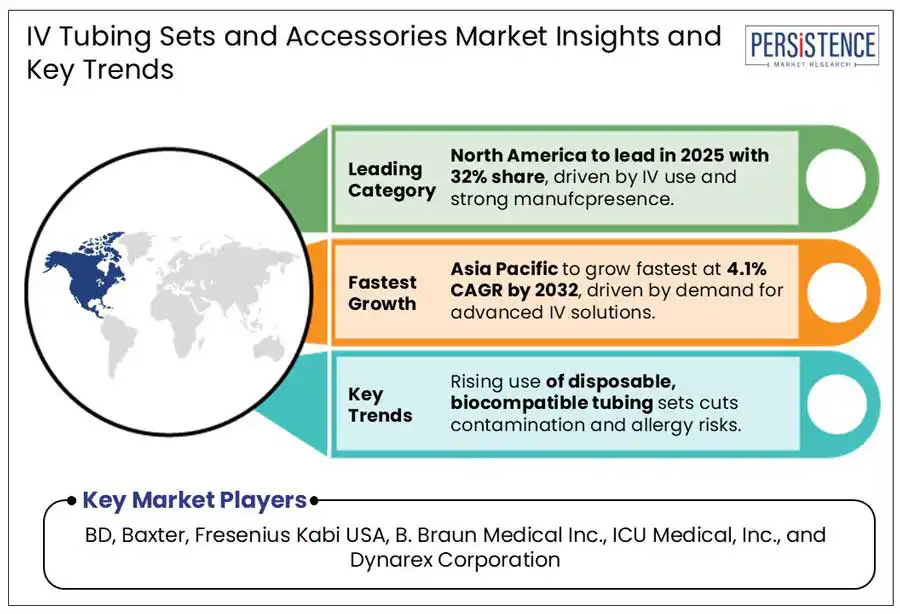
North America is projected to dominate the global market in 2025. Rising burden of chronic diseases, a high rate of hospital admissions and intravenous procedures, and a strong presence of leading manufacturers continue to drive the demand for IV products in North America. A recent study shows 83% of United States medical patients received some sort of IV therapy or infusion as of 2019. The growing incidence of chronic illnesses such as cancer, diabetes, and cardiovascular diseases further contributes to the growing use of intravenous therapies.
Europe is expected to hold over 25% of the global market share in 2025. A key driver of the rising demand within the market is the availability of highly qualified and competent specialists and technicians to administer IV administration devices.
The majority of regional producers offer reimbursement for different IV administration regimens. Since there are specific guidelines to follow while using IV tubing sets, the availability of qualified technicians attracts a large number of patients for surgeries, which fosters future growth for the overall market. Additionally, market growth is fuelled by the region's constantly changing reimbursement environment for IV tubing sets and the rising prevalence of chronic diseases.
Asia Pacific market is projected to be highly lucrative for the IV product manufacturers during the forecast period. The demand for IV tubing sets and accessories is growing significantly as a result of growing preference for minimally invasive procedures and the availability of technologically superior IV administration sets. A sharp rise in emergency care services and surgical procedures in countries such as India and China further accelerates the use of IV products.
Moreover, government-led healthcare expansion programs such as Ayushman Bharat in India and China’s Healthy China 2030 initiative are expanding access to quality care, including IV therapy. This growth is further supported by localized manufacturing, a cost-effective distribution network, and strategic collaborations with public health sectors to meet large-scale demand.
IV tubing set producers prioritise employing high-quality components to develop products that are reliable and simple to use in a range of health care settings. Partnerships with distributors as well as other channel partners ensure continuous product availability and increase the product's geographic reach as well as its applicability in non-traditional end-use environments. Manufacturers use a variety of key strategies to enhance product sales across different geographies, including infrastructure investments, new product introductions, and the introduction of new technologies.
The global market is set to reach US$ 1.3 Bn in 2025.
The market is projected to record a CAGR of 3.5% during the forecast period from 2025 to 2032.
Increasing demand for healthcare services, advancements in medical technologies, and a growing focus on patient safety and comfort during intravenous therapy is expected to drive the global market.
BD, Baxter, Fresenius Kabi USA, B. Braun Medical Inc., ICU Medical, Inc., and Dynarex Corporation are a few leading players.
North America is projected to dominate the global market in 2025.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author