ID: PMRREP3774| 199 Pages | 2 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

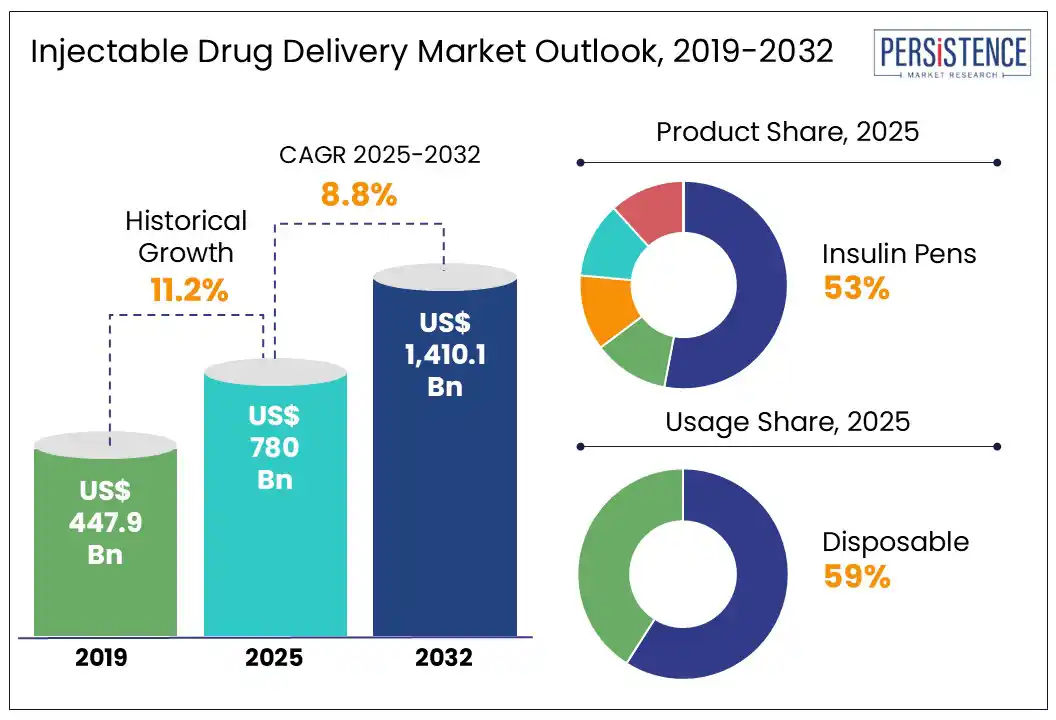
The injectable drug delivery market size is likely to be valued at US$ 780.0 Bn in 2025 and is estimated to reach US$ 1,410.1 Bn in 2032, growing at a CAGR of 8.8% during the forecast period 2025-2032.
Injectable drug delivery is entering a transformative phase, propelled by biologic innovation, digital health integration, and shifting patient behaviors. As chronic diseases become increasingly prevalent, injectables are emerging as the preferred mode of administration for their rapid bioavailability. The market is being reengineered for precision, comfort, and convenience with the launch of smart autoinjectors and wearable devices. Rising demand for the smooth delivery of high-viscosity biologics over extended periods is expected to create new opportunities.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Injectable Drug Delivery Market Size (2025E) |
US$ 780.0 Bn |
|
Market Value Forecast (2032F) |
US$ 1,410.1 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
8.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
11.2% |
Increasing prevalence of diabetes, cancer, and autoimmune disorders is directly augmenting demand for injectable drug delivery systems due to the nature of these diseases and the therapies used to treat them. In 2024, for example, more than 540 Mn people globally were living with diabetes, according to the International Diabetes Federation. The number is expected to surpass 640 Mn by 2030. Injectable insulin and GLP-1 receptor agonists such as semaglutide have become standard treatment options. Hence, companies are extending production of autoinjectors and wearable injectors to facilitate self-administration.
In oncology, injectable biologics such as checkpoint inhibitors and monoclonal antibodies dominate cancer immunotherapy pipelines. New cancer cases are predicted to surge from 20 Mn in 2022 to over 35 Mn by 2050, found the World Health Organization (WHO). Therefore, injectable drug formats remain essential due to their ability to bypass the gastrointestinal tract and deliver rapid, systemic effects. Companies are also launching subcutaneous formulations of drugs traditionally given intravenously such as Roche’s Phesgo, to enhance convenience.
The complexity of manufacturing injectable drug delivery systems is creating significant supply chain constraints, particularly as demand rises for biologics and self-administered therapies. These systems require high-precision engineering, sterile fill-finish operations, and rigorous quality control. All of these tend to lengthen production timelines and raise costs. Start-ups and small-scale biotech firms developing novel injectables often face significant hurdles in scaling up.
It is because few contract manufacturers have the specialized infrastructure to handle complex devices such as wearable injectors or dual-chamber syringes. This further creates a reliance on a limited pool of CDMOs, extending lead times and raising costs. As per a recent study, the average lead time for sterile injectable fill-finish slots at key CDMOs extended beyond 18 months in 2023, delaying market entry for several therapies.
The accelerating trend toward self-administration is fundamentally boosting the injectable drug delivery market growth, states Persistence Market Research. It is creating demand for patient-centric devices that prioritize ease of use, safety, and convenience. With chronic diseases such as rheumatoid arthritis and diabetes requiring long-term treatment, patients prefer administering medications at home rather than visiting clinics. This shift has led to a surge in the adoption of self-injectors that are specifically designed to facilitate accurate, consistent dosing with minimal medical supervision.
Wearable injectors are gaining impetus due to demand for at-home administration of biologics and high-viscosity drugs. The growth of digital health integration is also creating lucrative opportunities. Connected injectors equipped with Bluetooth are enabling dose-tracking and real-time adherence monitoring through mobile apps. This convergence of drug delivery and digital health is not only empowering patients but also attracting interest from providers seeking value-based care models.
By product, the market is divided into self-injectors, needle-free injectors, auto injectors, wearable injectors, and insulin pens. Among these, insulin pens are predicted to hold nearly 53.0% of the injectable drug delivery market share in 2025 with their ability to address both clinical and behavioral barriers associated with conventional vial-and-syringe methods. These devices offer improved dose accuracy, portability, and ease of use. These features make them ideal for patients with limited dexterity or visual impairments, which is a key concern as global diabetes prevalence escalates.
Auto injectors, on the other hand, are likely to witness steady growth through 2032 due to their ability to provide a controlled, patient-friendly solution for self-administering biologics. These devices eliminate the requirement for manual needle handling, thereby reducing user error and simplifying complex dosing regimens. Demand has been particularly high in therapeutic areas such as rheumatoid arthritis, where drugs such as adalimumab are offered in autoinjector formats.
In terms of usage, the market is bifurcated into disposable and reusable. Out of these, disposable injectors are anticipated to account for about 59.0% of share in 2025 due to their ability to ensure sterility, reduce contamination risks, and offer a single-use solution. These devices are suitable for self-administration settings and public health environments as these do not require complex cleaning or maintenance. Another key factor pushing their adoption is the surge in biologic therapies requiring subcutaneous or intramuscular administration.
Reusable injectors are seeing a decent growth rate owing to their long-term cost efficiency, sustainability benefits, and adaptability for frequent-use therapies. These devices significantly reduce per-dose costs over time, making them an attractive solution for patients requiring daily or weekly injections. These are being extensively used for insulin, fertility hormones, and human growth hormone treatments. Their ability to provide high injection comfort and superior dose personalization is also expected to boost demand.
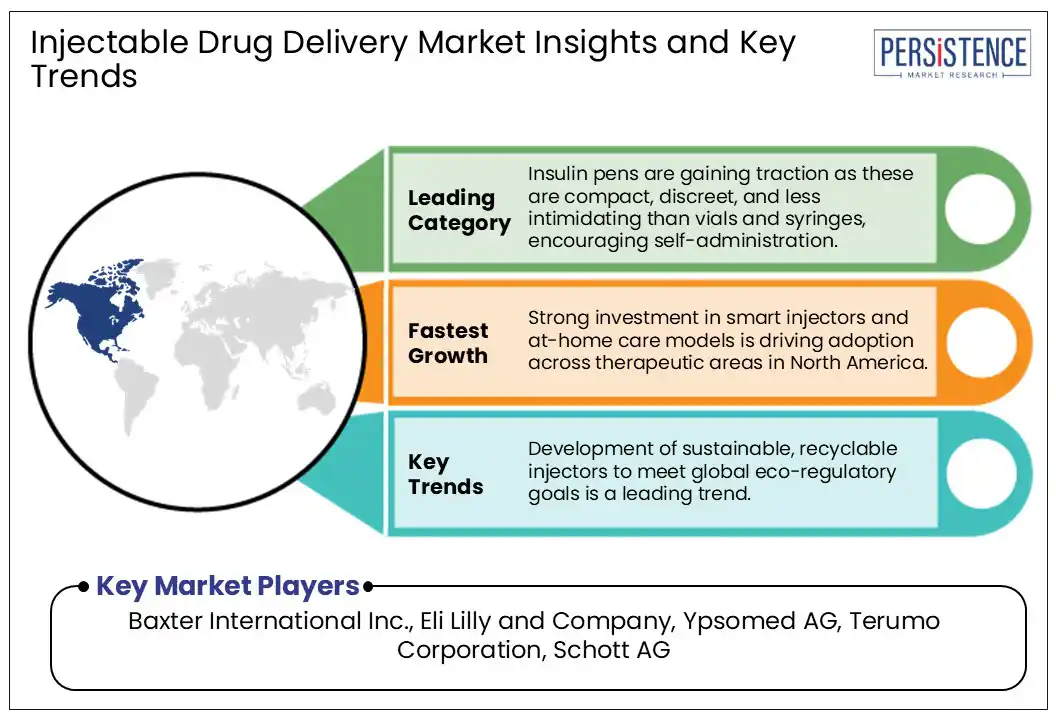
In 2025, North America is predicted to account for approximately 40.0% of share due to rising demand for self-administered therapies, particularly for chronic conditions such as diabetes and cancer. The U.S. injectable drug delivery market currently leads North America, owing to the country’s high biologics consumption and robust research and development pipelines. As per the IQVIA Institute, in 2024, over 60% of the 20 top-tier drugs in the U.S. by sales were biologics, most of which require parenteral administration. This surge is propelling developments in devices such as autoinjectors and prefilled syringes to enhance dosing accuracy and patient adherence.
The U.S. Food and Drug Administration’s (FDA) streamlined regulatory pathways for combination products and digital health integrations have also encouraged pharma-device partnerships. In 2023, for instance, Eli Lilly received FDA approval for its Tempo Smart Button. The device attaches to insulin pens and tracks dosage, reflecting the shift toward connected, patient-friendly injectables. Similarly, Amgen’s Enbrel and Neulasta have been widely adopted with on-body injector systems, reducing hospital visits and enabling at-home treatment. These trends are further reinforced by payers and providers advocating value-based care models that prioritize home-based delivery.
Europe is being pushed by high demand for biologics and self-administration tools, especially in home healthcare. Germany leads the charge, followed by key contributions from the U.K., France, and Italy. Among delivery modes, skin-targeted subcutaneous injectables remain the most preferred due to rapid chronic condition management. Technological innovations are also accelerating adoption.
Prefilled syringes, auto-injectors, wearables, microneedles, and needle-free systems are gaining traction in Europe. Auto-injectors are in high demand, especially for rheumatoid arthritis and multiple sclerosis management. The booming GLP1 injectable category is also reshaping fill-finish infrastructure. Domestic Contract Development and Manufacturing Organizations (CDMOs) such as Italy’s BSP Pharmaceuticals have significantly scaled capacity to support drugs, including Eli Lilly’s Zepbound.
Asia Pacific is experiencing a rapid transformation, spurred by surging demand for chronic disease management and strong innovation ecosystems. India stands out as a key growth engine due to government initiatives such as Make in India for pharmaceutical manufacturing and emerging academic-industry collaborations. IIT Guwahati, for example, is actively working on nanomaterial-enabled delivery mechanisms, while IIT Bombay recently introduced a needle-free shock wave injector. These developments indicate a move toward cost-effective and patient-friendly alternatives to conventional injections.
Japan and South Korea are responding to their aging populations with injectable therapies designed for improved usability. Japan-based companies are developing subcutaneous Alzheimer’s treatments aimed at reducing hospital dependency. Regulatory innovation is further bolstering the market as Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) extended its presence across Asia Pacific to facilitate regional clinical trial cooperation. South Korea’s Ministry of Food and Drug Safety (MFDS) also achieved WHO Listed Authority status in 2024, accelerating product approvals.
The injectable drug delivery market consists of various pharmaceutical giants, medical device manufacturers, and niche biotech firms. Key companies are strengthening their market position by offering novel prefilled syringes and auto-injectors, often in partnership with drug manufacturers. Pharmaceutical companies are integrating drug-device combinations, especially for self-administration of chronic therapies such as cancer and diabetes. Their focus lies in enhancing drug adherence through patient-centric designs, including on-body injector platforms. Competition is intensifying in the biologics and biosimilars space, where injectable formulations require precise delivery systems.
The market is projected to reach US$ 780.0 Bn in 2025.
Rising prevalence of chronic diseases and increasing patient preference for self-administration are the key market drivers.
The market is poised to witness a CAGR of 8.8% from 2025 to 2032.
Surge in wearable injectors and increasing use of microneedle technologies are the key market opportunities.
Baxter International Inc., Eli Lilly and Company, and Ypsomed AG are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Technology
By Usage
By Indication
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author