ID: PMRREP34600| 200 Pages | 3 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

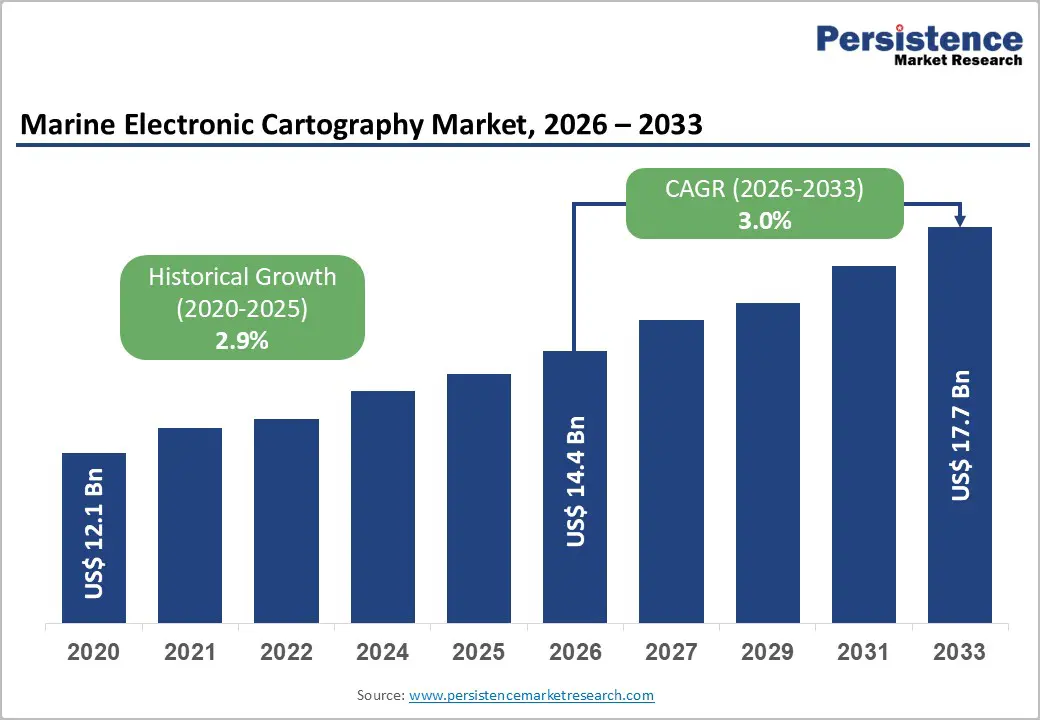
The global monoclonal antibodies market is estimated to grow from US$ 310.8 Bn in 2026 to US$ 726.8 Bn by 2033. The market is projected to grow at a CAGR of 12.9% from 2026 to 2033.
The global monoclonal antibodies industry is growing steadily, driven by rising demand for targeted therapies, increasing adoption of digital healthcare, and advancements in analytical and bioprocessing technologies. North America leads the market due to strong infrastructure, stringent regulatory standards, and high-quality manufacturing capabilities. The Asia-Pacific region is the fastest-growing region, driven by expanding healthcare facilities, supportive government initiatives, increasing patient awareness, and investments in advanced diagnostic and manufacturing solutions.
| Global Market Attributes | Key Insights |
|---|---|
| Monoclonal Antibodies Market Size (2026E) | US$ 310.8 Bn |
| Market Value Forecast (2033F) | US$ 726.8 Bn |
| Projected Growth (CAGR 2026 to 2033) | 12.9% |
| Historical Market Growth (CAGR 2020 to 2025) | 11.5% |
The global burden of cancer continues to escalate, driving demand for targeted biologics such as monoclonal antibodies (mAbs). According to the World Health Organization (WHO), in 2022 there were an estimated 20 million new cancer cases globally and 9.7 million cancer deaths, illustrating the substantial therapeutic need that targeted cancer therapies aim to address. Monoclonal antibodies are increasingly integral to oncology care due to their ability to selectively target tumor antigens, reduce off target toxicity, and improve survival outcomes compared with traditional chemotherapies. Additionally, mAb therapies are widely used for autoimmune conditions such as rheumatoid arthritis and inflammatory disorders, where precision immune modulation can significantly improve disease control.
Beyond oncology and autoimmune diseases, monoclonal antibodies are also employed against certain infectious diseases. The increasing emphasis on biologics during the COVID19 pandemic highlighted mAbs as effective antiviral tools, including emergency use authorizations for SARS CoV 2 neutralizing antibodies. Although not a replacement for vaccines, these therapies contribute to the management of acute disease, particularly in high-risk populations. Combined with aging populations and rising prevalence of chronic diseases, these factors sustain robust demand for mAbs as targeted therapies across therapeutic areas, thereby driving market growth.
Monoclonal antibody therapies are expensive to develop and produce, posing a significant market restraint. The development of a novel therapeutic mAb can exceed $2 billion USD, driven by extensive R&D, multi phase clinical trials, and regulatory requirements. Manufacturing biologics such as mAbs demands highly specialized infrastructure (e.g., mammalian cell culture systems, stringent bioreactor controls), sophisticated purification processes, and rigorous quality testing to ensure product consistency and safety. These complexities substantially increase both capital expenditures and operating costs.
From a commercial perspective, high development and production costs translate into expensive therapies for patients. Many therapeutic mAbs are priced above $100,000 per patient per year, making them less accessible, particularly in low and middle-income regions where health systems and insurance coverage may be limited. The combination of high R&D outlays, lengthy development timelines, and sophisticated manufacturing requirements creates barriers to entry for smaller biotech firms, slows pipeline progression, and limits affordability, restricting broader adoption and dampening market growth in cost-sensitive environments.
The next frontier in antibody therapeutics lies in bispecific and multispecific antibodies (MsAbs) that can simultaneously bind two distinct targets, offering enhanced therapeutic efficacy in complex diseases. Bispecific antibody formats expand the functional capabilities of traditional monoclonal antibodies by engaging multiple antigens or immune pathways with a single molecule. This enables, for example, the direct recruitment of T cells to tumor cells to enhance immune activation, representing a promising approach in oncology and beyond.
The FDA has already approved more than a dozen bispecific antibodies, including agents targeting CD20 and CD3 for lymphoma, confirming clinical feasibility and commercial potential. Additionally, research into multispecific constructs and engineered antibody scaffolds is expanding, with platforms designed to enhance binding, reduce resistance, and improve clinical outcomes in cases where single-target therapies are limited. As the technology matures and regulatory pathways evolve, these next-generation antibody formats offer a significant opportunity to address unmet medical needs and to unlock new therapeutic categories, substantially expanding the monoclonal antibody market.
Human source occupies 52.3% share of the global market in 2025, because they offer reduced immunogenicity and improved safety profiles, which are critical for chronic and life threatening conditions such as cancer and autoimmune diseases. Fully human antibodies are structurally most similar to naturally occurring human antibodies, minimizing the risk of immune reactions that can limit efficacy or cause adverse events, a key regulatory and clinical priority.
Moreover, advancements in technologies like transgenic animal models and phage display libraries have expanded the pipeline of fully human monoclonal antibodies, improving binding specificity and therapeutic durability. These innovations support broader clinical acceptance and higher uptake across multiple therapeutic areas, reinforcing the human segment’s leadership in the monoclonal antibodies market.
In vitro production methods dominate monoclonal antibody manufacturing because they provide scalable, controlled, and reproducible production compared with in vivo (animal-based) approaches. Controlled cell culture systems, most commonly using mammalian cells such as Chinese hamster ovary (CHO) lines, enable tight regulation of growth conditions and consistent antibody quality, which is essential for clinical and commercial supply. This advantage has led the in vitro segment to account for an estimated 60–75% share of the production-type market in 2024–2025.
In vitro systems also facilitate regulatory compliance, reduced contamination risk, and cost-effective scale-up to meet global demand for therapeutic mAbs. They support advanced bioprocessing technologies such as single-use bioreactors and continuous manufacturing, which further enhance yield and production efficiency for large-volume biologics. These operational strengths make in vitro production the preferred choice for most commercial monoclonal antibody manufacturing.
North America dominates the monoclonal antibodies market with 45.8% share in 2025, due to unparalleled biomedical research capacity and high disease burden, emphasizing targeted therapies. The U.S. accounts for a large proportion of global cancer cases, over 2 million new diagnoses projected in 2025 alone, fueling demand for biologics like mAbs for oncology care. The region’s robust healthcare infrastructure and advanced R&D ecosystem are supported by major public institutions such as the National Institutes of Health (NIH) and the National Cancer Institute (NCI), which coordinate extensive research and clinical trial networks. Additionally, high healthcare spending and comprehensive reimbursement frameworks enable widespread adoption of innovative therapies, reinforcing North America’s leading share of the global market.
Europe is a significant market for monoclonal antibodies due to its robust healthcare systems, broad adoption of advanced therapeutics, and a structured regulatory environment. Countries such as Germany, France, and the United Kingdom have well-established clinical infrastructures and comprehensive public health coverage that ensure access to biologic treatments. The regional market is driven by increasing prevalence of chronic diseases, including cancer and autoimmune disorders and rising patient awareness of targeted therapies. Europe’s pharmaceutical sector benefits from coordinated clinical trial activity and regulatory oversight by authorities such as the European Medicines Agency (EMA), promoting rigorous safety standards and the adoption of innovation. With a substantial share (~28–30% globally), Europe’s reimbursement systems and research networks play a pivotal role in integrating mAb therapies into routine care.
The Asia Pacific region is the fastest-growing monoclonal antibodies market due to rapid healthcare development, expanding biopharmaceutical capacity, and rising disease prevalence. Countries like China, India, and Japan are increasing healthcare investments and building manufacturing infrastructure for biologics, supported by evolving regulatory frameworks that streamline approvals for new therapies. The region’s large and aging population contributes to a growing patient pool with chronic conditions such as cancer and autoimmune disorders, driving demand for targeted mAb treatments. Additionally, government initiatives and expansions in clinical research increase local R&D and help reduce treatment costs, thereby boosting access and adoption. These factors underpin Asia-Pacific’s robust compound annual growth rate (often outpacing Western regions), making it the fastest-growing regional market for monoclonal antibodies.
The monoclonal antibodies market is highly competitive, led by companies like Roche, AbbVie, Amgen, and Johnson & Johnson. Firms compete on innovation, pipeline diversity, and biologic engineering. Strategic collaborations, biosimilar launches, and global expansion drive market positioning, while R&D investment and regulatory approvals determine long-term leadership.
The global monoclonal antibodies market is projected to be valued at US$ 310.8 Bn in 2026.
Rising demand for targeted therapies, technological advances, personalized medicine adoption, and growing biopharmaceutical investments drive growth.
The global monoclonal antibodies market is poised to witness a CAGR of 12.9% between 2026 and 2033.
Next-generation antibodies, bispecifics, AI-enabled optimization, rare disease therapies, scalable manufacturing, and emerging market expansion offer opportunities.
Novartis AG, Pfizer Inc, GlaxoSmithKline plc, Amgen Inc., Merck & Co., Inc., Daiichi Sankyo Company, Limited.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Source Type
By Production Type
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author