ID: PMRREP33788| 210 Pages | 13 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

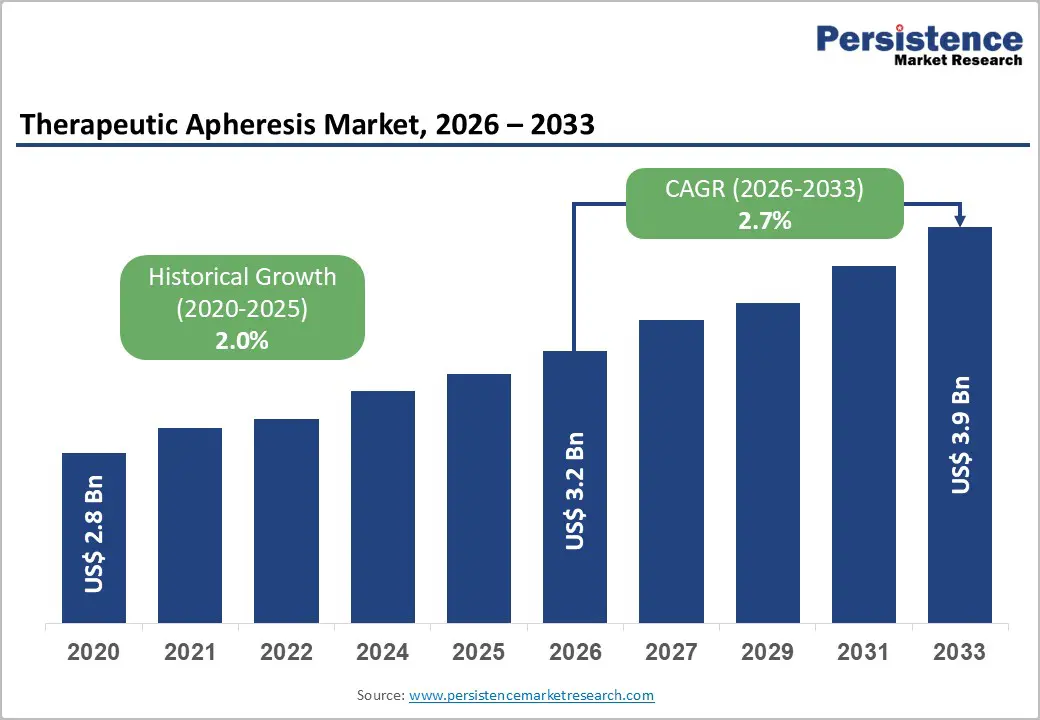
The global therapeutic apheresis market size is likely to be valued at US$3.2 billion in 2026 and projected to reach US$3.9 billion by 2033, growing at a CAGR of 2.7% during the forecast period from 2026 to 2033. Therapeutic apheresis, which is alternatively spelled "apheresis" or "plasmapheresis," is a medical procedure in which certain blood components are extracted extracorporeally, separated, and returned to treat a variety of medical conditions. The aforementioned method for selectively purifying blood is crucial for the management of autoimmune diseases, neurological disorders, and specific hematologic conditions. Therapeutic apheresis employs specialized equipment to selectively remove harmful components, thereby facilitating the patient's restoration to purified blood.
This specific medical intervention has demonstrated significant efficacy in alleviating symptoms and improving overall health in individuals with a range of health issues. Numerous factors contribute to the expansion of the therapeutic apheresis market worldwide. The rising incidence of autoimmune disorders and hematologic diseases, in conjunction with technological advances in the medical field, stimulates the need for therapeutic apheresis procedures.
| Key Insights | Details |
|---|---|
|
Therapeutic Apheresis Market Size (2026E) |
US$3.2 Bn |
|
Market Value Forecast (2033F) |
US$3.9 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
2.7% |
|
Historical Market Growth (CAGR 2020 to 2025) |
2.0% |
The rapid growth of CAR-T and broader cell and gene therapy ecosystems is emerging as a powerful indirect driver for the therapeutic apheresis market, particularly through rising demand for leukapheresis and immune-cell collection platforms. CAR-T therapy begins with the extraction of a patient’s T-cells, a highly specialized leukapheresis process that requires advanced apheresis systems capable of delivering high cell yield, purity, and viability. As the number of approved CAR-T products and late-stage pipelines continues to expand across hematological malignancies and, increasingly, solid tumors, hospitals and cell therapy centers are scaling up apheresis capacity to meet growing procedural volumes.
In parallel, pharmaceutical companies and contract development and manufacturing organizations (CDMOs) are investing heavily in cell-processing infrastructure, leading to standardization of apheresis protocols and greater reliance on validated, regulatory-compliant devices. Unlike conventional therapeutic plasma exchange, leukapheresis for cell therapies demands tighter control over flow rates, anticoagulation, and collection consistency, driving adoption of next-generation, automated apheresis platforms and single-use disposables. This trend is also accelerating long-term service contracts, consumables demand, and device upgrades.
Additionally, the geographic expansion of CAR-T programs into emerging markets is creating new demand pockets for apheresis equipment in tertiary hospitals and specialty oncology centers. Training programs for clinicians and technicians are increasingly integrated with cell therapy rollouts, further embedding apheresis into oncology care pathways. As autologous and allogeneic cell therapies progress toward earlier lines of treatment, apheresis is transitioning from a supportive tool to a foundational enabling technology within the evolving advanced therapy landscape.
Procedure-induced hemodynamic instability remains a critical restraint in the therapeutic apheresis market, particularly affecting adoption among vulnerable patient populations. During apheresis, rapid extracorporeal blood circulation and plasma volume shifts can trigger acute hypotension, especially in patients with compromised cardiovascular function or low baseline blood pressure. The use of citrate as an anticoagulant, while effective in preventing clotting, often leads to citrate toxicity by chelating calcium, resulting in hypocalcemia. This can cause symptoms ranging from mild tingling and muscle cramps to severe arrhythmias, seizures, or cardiac instability if not promptly managed. Electrolyte imbalances, including fluctuations in potassium and magnesium levels, further complicate clinical management during prolonged or repeated sessions.
These physiological risks are particularly pronounced in critically ill patients, pediatric populations, and the elderly, who often have reduced physiological reserves and multiple comorbidities. In such cases, clinicians may hesitate to recommend therapeutic apheresis or opt for alternative pharmacological treatments with more predictable safety profiles. Additionally, the need for continuous monitoring, calcium supplementation, and emergency preparedness increases procedural complexity and resource utilization. This not only limits the pool of eligible patients but also raises operational costs for healthcare providers. As a result, concerns over hemodynamic stability significantly restrain broader adoption of therapeutic apheresis, especially in non-ICU settings and resource-constrained healthcare environments.
The integration of telemedicine and remote monitoring with therapeutic apheresis devices represents a significant advancement in patient care, particularly for regions with limited access to specialized healthcare. Traditionally, apheresis procedures require hospital- or clinic-based supervision due to the need for trained clinicians and real-time monitoring of patient vital signs. However, by leveraging connected devices, healthcare providers can now monitor procedures remotely, enabling virtual supervision by experts located off-site.
This approach enhances accessibility for patients in rural or underserved areas, reducing the need for travel and minimizing treatment delays. Remote monitoring systems can track critical parameters such as blood pressure, heart rate, and flow rates, while AI-enabled alerts can immediately notify clinicians of potential complications, improving safety outcomes.
Additionally, integrating telemedicine enables real-time consultations, procedural guidance, and follow-up care, creating a seamless continuum of care. Healthcare facilities can also optimize staff utilization, as fewer on-site specialists are required per procedure. Over time, this model may support home-based apheresis programs for stable chronic patients, further decentralizing care.
Overall, integrating telemedicine and remote monitoring improves patient convenience and safety and expands the market reach of therapeutic apheresis technologies, offering a scalable, future-ready solution for modern healthcare.
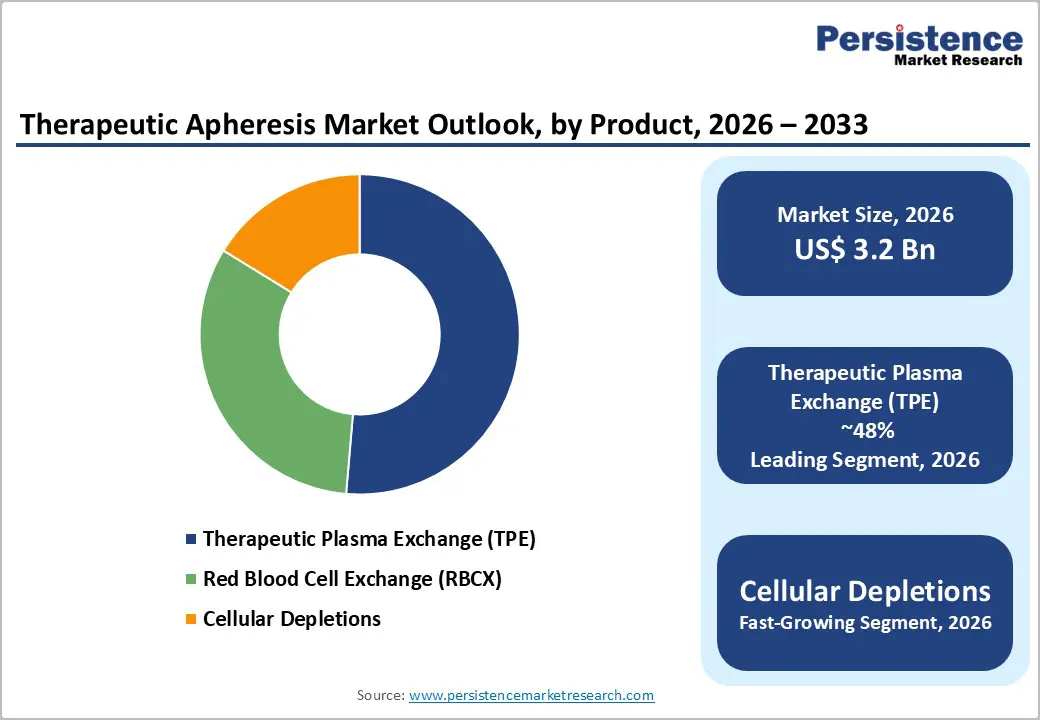
Therapeutic Plasma Exchange (TPE) accounts for the highest market share among apheresis procedures due to its broad clinical applicability, established efficacy, and strong guideline support. TPE involves removing plasma containing pathogenic substances, such as autoantibodies, immune complexes, or toxins, and replacing it with donor plasma or albumin. This mechanism makes it effective across a wide range of autoimmune, neurological, and hematological disorders, including myasthenia gravis, Guillain-Barré syndrome, thrombotic thrombocytopenic purpura, and hyperviscosity syndromes. The procedure is highly recommended in international clinical guidelines, which reinforces physician confidence and encourages routine adoption in hospitals and specialized centers.
In contrast, Red Blood Cell Exchange (RBCX) is primarily limited to hemoglobinopathies such as sickle cell disease, and Cellular Depletions (e.g., leukapheresis) are typically reserved for the targeted removal of specific blood components in niche conditions. These specialized indications limit their overall procedural volumes and market penetration. Furthermore, TPE procedures benefit from recurring demand for chronic conditions, driving both device and consumable sales. Technological advancements in automation, safety features, and efficiency have also made TPE easier to perform, reducing complications and increasing patient throughput. Collectively, these factors position TPE as the leading procedure in the global therapeutic apheresis market.
Hematology represents the highest market share among therapeutic apheresis applications due to the high prevalence and procedural demand of blood-related disorders. Apheresis procedures such as Therapeutic Plasma Exchange (TPE), Red Blood Cell Exchange (RBCX), and leukapheresis are routinely employed in hematologic conditions, including thrombotic thrombocytopenic purpura (TTP), sickle cell disease, hyperviscosity syndromes, and leukemias. These disorders often require repeated or urgent interventions, resulting in consistently high procedure volumes compared to other therapeutic areas.
Moreover, hematologic indications are well-supported by clinical guidelines, which reinforces physician confidence in using apheresis as a standard treatment option. The broad applicability of these procedures across acute and chronic hematologic conditions ensures continuous demand for both apheresis devices and consumables, such as plasma filters, tubing sets, and replacement fluids.
In contrast, applications in neurology, nephrology, rheumatology, and oncology are comparatively limited. Neurological and rheumatological uses, while growing, cater to smaller patient populations, and oncology mainly involves cell collection for stem cell or CAR-T therapies, which are specialized procedures with lower overall procedural volumes.
Additionally, technological advancements improving efficiency, safety, and automation have further facilitated the widespread adoption of apheresis in hematology. Collectively, the combination of high prevalence, recurring procedural need, and guideline-backed clinical use makes hematology the dominant application segment in the global therapeutic apheresis market.
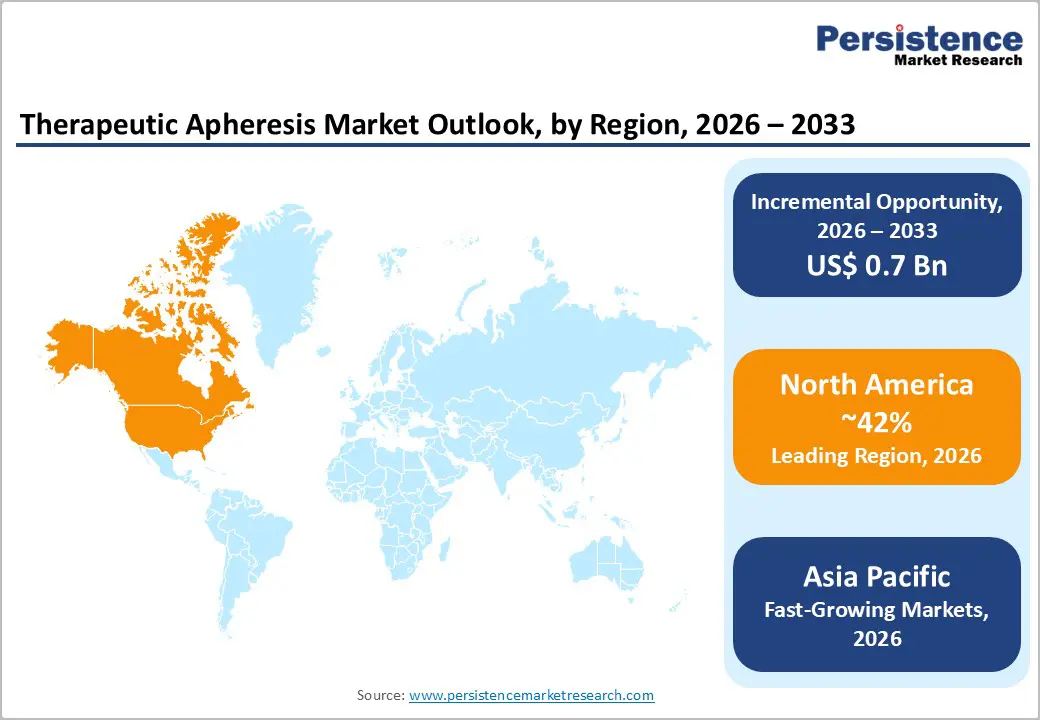
North America leads the global therapeutic apheresis market, driven by advanced healthcare infrastructure, high disease prevalence, and widespread adoption of innovative technologies. The region benefits from well-established hospitals and specialty clinics equipped with state-of-the-art apheresis devices, trained healthcare professionals, and comprehensive patient monitoring systems. Therapeutic Plasma Exchange (TPE) and Red Blood Cell Exchange (RBCX) remain widely used for hematologic, neurological, and autoimmune disorders, supported by strong clinical guidelines and insurance reimbursement policies.
In the U.S., the market is particularly robust due to rising cases of autoimmune diseases, chronic blood disorders, and hematologic malignancies. According to the American Society for Apheresis, thousands of procedures are performed annually across tertiary care centers. Favorable reimbursement frameworks, high patient awareness, and increasing adoption of novel automated and AI-enabled apheresis devices contribute to market growth. Additionally, the U.S. is at the forefront of integrating apheresis with emerging therapies such as CAR-T cell and stem cell treatments, expanding clinical indications and procedural volumes.
Overall, North America’s leadership is reinforced by consistent technological innovation, strategic investments by key device manufacturers, and a mature healthcare ecosystem that supports both standard and advanced apheresis procedures, making it the largest and most sophisticated market globally.
The Asia-Pacific region is emerging as the fastest-growing market for therapeutic apheresis, driven by increasing healthcare infrastructure, rising prevalence of chronic and autoimmune disorders, and growing awareness among patients and clinicians. Countries such as China, Japan, India, and South Korea are witnessing significant investments in modern hospitals, specialty clinics, and blood centers, enabling wider access to advanced apheresis procedures like Therapeutic Plasma Exchange (TPE), Red Blood Cell Exchange (RBCX), and leukapheresis.
The rising incidence of hematologic and neurological disorders, along with a growing geriatric population, is creating higher procedural demand. The region also benefits from expanding medical tourism, where patients from neighboring countries seek cost-effective, high-quality apheresis treatment. Technological adoption is increasing, with automated and portable apheresis devices being introduced to improve safety, efficiency, and patient throughput.
Additionally, government initiatives, funding support, and collaborations between local hospitals and international device manufacturers are facilitating market growth. Despite challenges such as high procedure costs and limited trained personnel in some areas, the combination of unmet clinical needs, expanding infrastructure, and increasing awareness positions Asia-Pacific as a high-potential growth market in the global therapeutic apheresis landscape.
The therapeutic apheresis market is highly competitive, driven by continuous technological innovation, device automation, and expansion of consumables portfolios. Key players focus on strategic initiatives such as product launches, partnerships, mergers, and regional expansion to strengthen market presence. Companies compete on parameters like device efficiency, safety features, ease of use, and procedure versatility across multiple indications. Recurring revenue from disposables and maintenance services adds to competitive dynamics.
The global therapeutic apheresis market is projected to be valued at US$3.2 Bn in 2026.
Development of automated, efficient, and AI-enabled apheresis devices enhances safety, reduces procedure time, and improves patient outcomes, encouraging adoption.
The global market is poised to witness a CAGR of 2.7% between 2026 and 2033.
Apheresis for harvesting lymphocytes and mononuclear cells for CAR-T and stem cell therapies presents high-value growth potential.
Asahi Kasei Medical Co., Haemonetics Corporation, Miltenyi Biotec, Terumo BCT Inc., and others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Procedure Type
By Technology
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author