ID: PMRREP12868| 199 Pages | 30 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

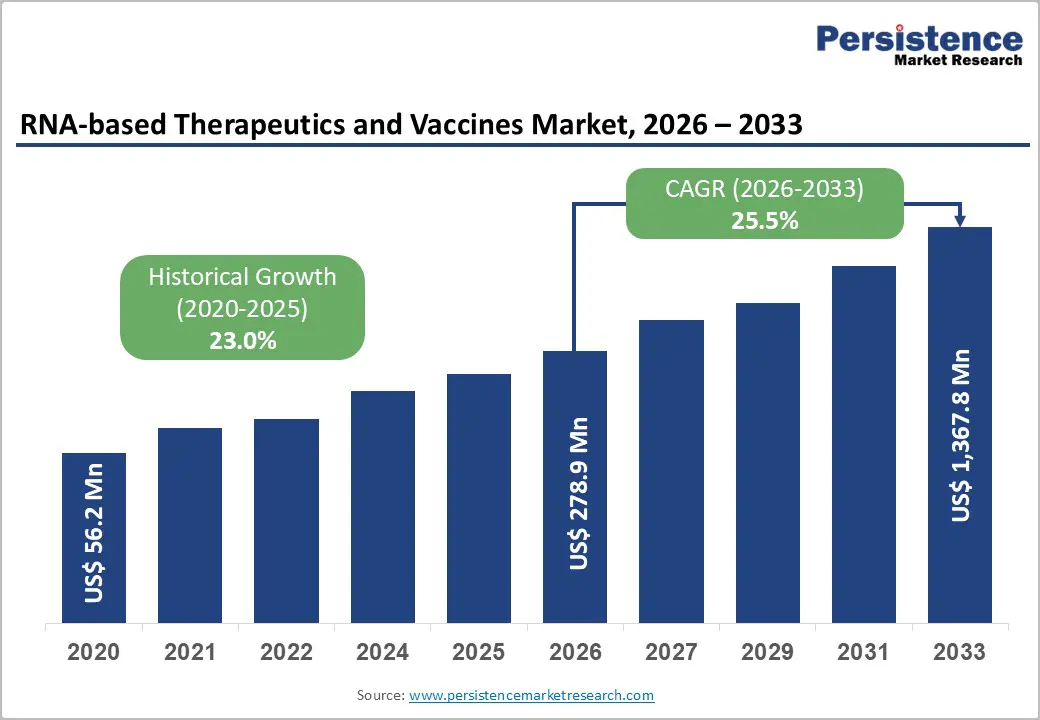
The global RNA-based therapeutics and vaccines market is estimated to grow from US$ 278.9 Mn in 2026 to US$ 1,367.8 Mn by 2033. The market is projected to record a CAGR of 25.5% during the forecast period from 2026 to 2033.
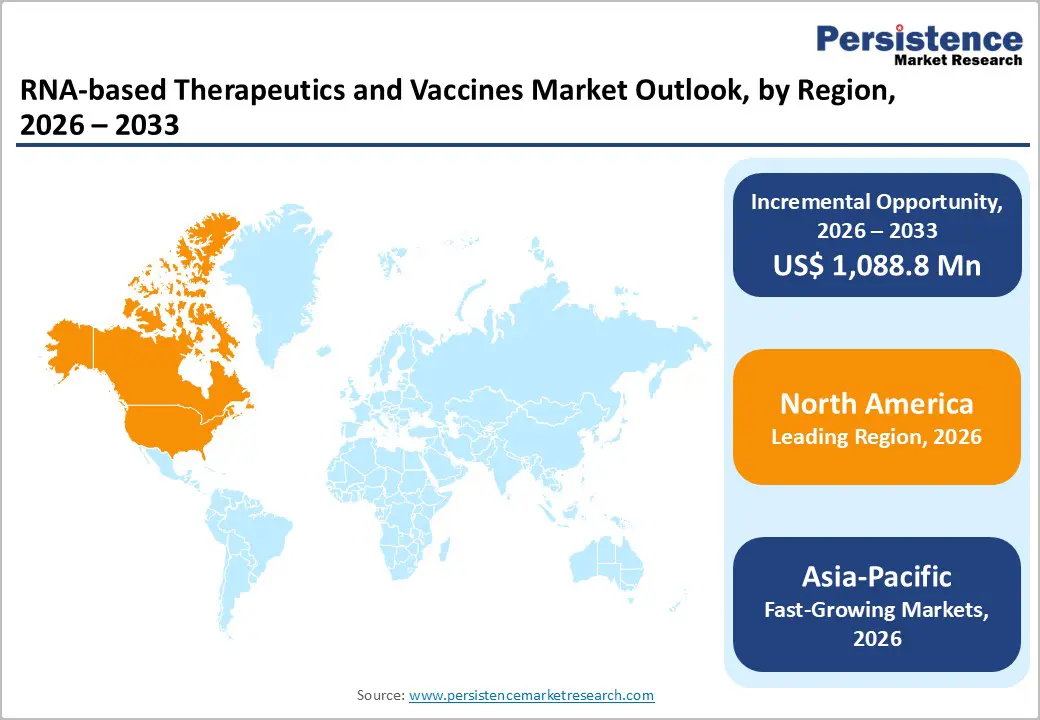
The global RNA-based therapeutics and vaccines market is expanding steadily, driven by adoption of mRNA platforms, strong clinical pipelines, rising infectious and genetic disease burden, and technological advances in delivery systems. North America leads due to robust R&D, funding, and regulation, while Asia-Pacific grows fastest, supported by manufacturing scale, government initiatives, and expanding patient access.
| Global Market Attributes | Key Insights |
|---|---|
| Global RNA-based Therapeutics and Vaccines Market Size (2026E) | US$ 278.9 Mn |
| Market Value Forecast (2033F) | US$ 1,367.8 Mn |
| Projected Growth (CAGR 2026 to 2033) | 25.5% |
| Historical Market Growth (CAGR 2020 to 2025) | 23.0% |
Driver: Clinical Success of mRNA Vaccines and Therapies
The unprecedented clinical success of mRNA vaccines, particularly those developed for COVID-19, provides strong validation for RNA based therapeutic platforms. Large phase III clinical trials showed that mRNA 1273 (Moderna) demonstrated 94.1% efficacy in preventing symptomatic COVID-19 infection compared with placebo, with no significant safety concerns identified in tens of thousands of participants. Real-world evidence in over 136,000 individuals further confirmed high effectiveness, showing 86.1% to 93.3% reduction in SARS-CoV-2 infection and strong protection against hospitalization. These results have reinforced confidence in mRNA platforms across regulatory agencies and clinicians, underscoring the adaptability of the technology beyond a single pathogen to broader RNA therapies.
Building on these outcomes, mRNA technologies are now being explored for novel indications, including personalized cancer vaccines and treatments for genetic disorders. The strong efficacy and safety profile observed in public health-scale deployments have encouraged regulators and institutions to support broader RNA therapeutic programs. Continued demonstration of clinical benefit, real-world effectiveness, and expanded indication pipelines significantly drives investment and adoption in the RNA-based Therapeutics and Vaccines Market, making mRNA approaches a cornerstone of next-generation biologics development.
Restraints: High Development and Manufacturing Costs
Despite promising clinical outcomes, RNA-based therapeutic development entails high production and manufacturing costs that restrain broader adoption. Unlike traditional small molecules, RNA drugs require highly specialized vaccines and therapeutics manufacturing facilities with stringent quality control and cGMP-grade processes, significantly increasing capital and operating expenses. Further, RNA therapeutics often necessitate cold-chain logistics and ultra-low temperature storage to maintain molecular stability, adding substantial distribution and supply-chain costs that are especially burdensome in resource-limited settings. These factors collectively elevate the overall cost of goods and complicate scalability, often translating to high per-patient treatment expenses.
The intrinsic instability of RNA molecules also introduces formulation complexity and stringent process monitoring requirements, which further drive up early-phase development expenditures. For therapeutic developers, securing favorable reimbursement becomes challenging when pricing must reflect both the significant R&D outlay and the intricate manufacturing demands. These cost barriers can slow the pace of commercialization and dampen investment from smaller biotech firms that lack deep financial resources, representing a notable restraint in the RNA-based Therapeutics and Vaccines Market.
Opportunity: Expansion of Personalized and Precision RNA Medicines
A major strategic opportunity in the RNA-based therapeutics and vaccines market lies in the expansion of personalized and precision RNA medicines. RNA platforms are inherently adaptable due to their sequence-based design; changing the nucleotide sequence enables targeting of specific gene variants or neoantigens tailored to an individual’s disease profile. This precision capability is already being leveraged in investigational personalized mRNA cancer vaccines, such as those encoded to match tumor-specific mutations, and is supported by clinical pipeline activity for tailored oncology immunotherapies. The flexibility of RNA therapeutics extends further into rare genetic diseases by enabling the development of treatments that compensate for or silence pathogenic gene expressions, offering therapeutic avenues where traditional drugs have failed.
The rapid design and potential rapid manufacturing of RNA therapies, particularly with advances in delivery platforms and AI-assisted sequence optimization, could reduce development timelines and make patient-specific treatments more feasible. Coupled with genomic sequencing and precision medicine frameworks already promoted by health systems, this opportunity positions RNA therapeutics as a transformative modality that can address previously intractable diseases on an individualized basis, expanding market potential and clinical impact.
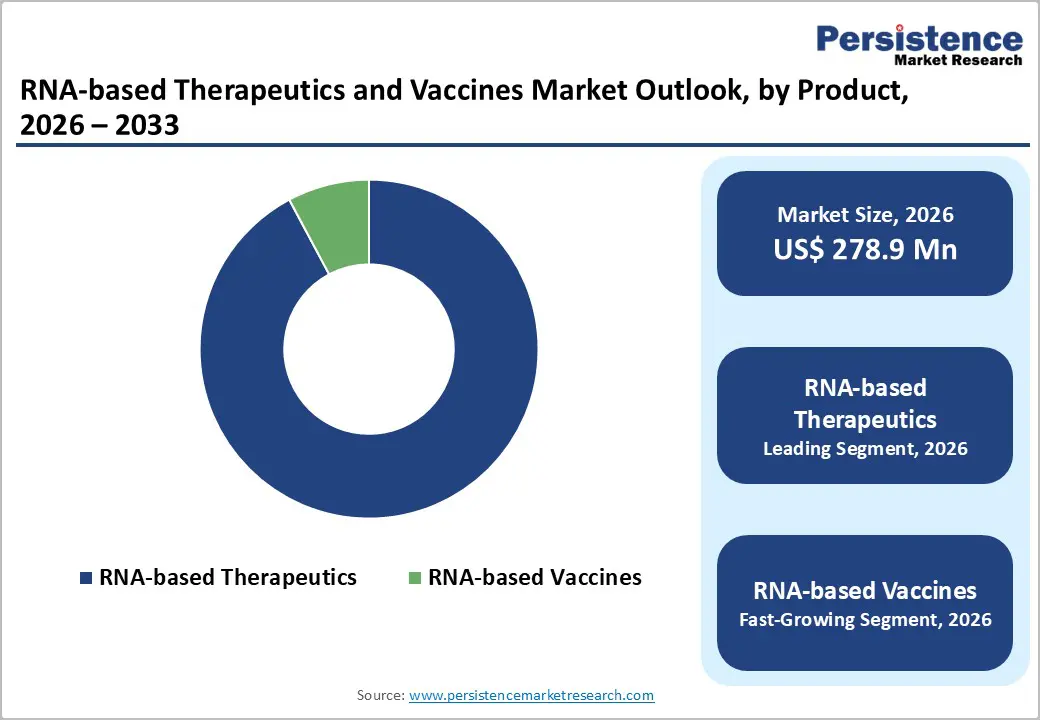
By Product, RNA-based Therapeutics Dominates the RNA-based Therapeutics and Vaccines Market
RNA-based Therapeutics occupies 92.2% share of the global market in 2025, because they address a broad spectrum of diseases beyond infectious vaccines. This category includes mRNA drugs, small interfering RNA (siRNA), and antisense oligonucleotides (ASOs), which can modulate gene expression and protein production in vivo, enabling treatment of genetic disorders, metabolic diseases, and cancer. Clinically approved therapeutics like Patisiran, Lumasiran, and Viltolarsen highlight their growing adoption. Regulatory approvals, coupled with sustained R&D investment in delivery technologies such as lipid nanoparticles, allow broader clinical application and revenue generation. While RNA vaccines focus mainly on prevention, therapeutics provide treatment options across multiple indications, expanding market share and making RNA therapeutics the dominant product segment.
By Indication Type, Infectious Diseases leads due to mRNA vaccine speed, effectiveness, and global adoption
Infectious diseases dominate the RNA-based therapeutics and vaccines market due to the proven effectiveness and rapid development potential of mRNA platforms. The COVID-19 pandemic demonstrated that mRNA vaccines could be designed, tested, and deployed globally within a year, highlighting unmatched speed and scalability. The World Health Organization notes RNA vaccines allow rapid candidate production critical for emerging pathogens. RNA vaccine pipelines are actively targeting influenza, RSV, HIV, cytomegalovirus, Zika, and rabies, emphasizing sustained focus on infectious disease prevention. Global health priorities, pandemic preparedness, and the widespread adoption of RNA vaccines contribute to infectious diseases capturing the largest market share among indications.
North America RNA-based Therapeutics and Vaccines Market Trends
North America leads the market with 46.3% share in 2025, due to its advanced biotechnology ecosystem, robust R&D infrastructure, and concentrated presence of major developers. The United States, in particular, leads with substantial contributions to mRNA vaccine development and commercialization for COVID-19 vaccines and other RNA platforms. This dominance is supported by strong federal funding, world-class academic institutions, and a regulatory framework (e.g., the FDA) that has authorized numerous RNA-based products. North American companies like Moderna and Pfizer-BioNTech have driven both innovation and adoption, supported by extensive clinical trial operations across all phases. The high concentration of expertise and capital continues to sustain leadership in RNA research and market share.
Europe RNA-based Therapeutics and Vaccines Market Trends
Europe represents a key region in the global RNA-based therapeutics and vaccines market due to its strong academic research base, collaborative clinical science, and supportive regulatory environment. Countries such as Germany, the U.K., and France are significant centers for genomic and RNA therapeutic research with active clinical trial portfolios. European regulators, including the European Medicines Agency (EMA), facilitate the approval and integration of innovative RNA modalities, contributing to region-wide growth and adoption. The region’s biotech ecosystem benefits from public-private partnerships and dedicated funding mechanisms that accelerate RNA drug development and deployment. Europe’s mature healthcare systems and investment in life sciences infrastructure help sustain its role as a major contributor to global RNA vaccine and therapeutic innovation, fostering both scientific advancement and market relevance.
Asia-Pacific RNA-based Therapeutics and Vaccines Market Trends
Asia-Pacific is the fastest-growing region in the RNA-based therapeutics and vaccines Market driven by expanding biotech infrastructure, rising government support, and increasing clinical research activity. Nations such as China, Japan, and South Korea are rapidly scaling RNA development through regulatory reforms, enhanced funding, and technology transfer initiatives that lower barriers for novel therapeutics. The increasing prevalence of infectious and chronic diseases across large populations also fuels demand for advanced RNA vaccines and therapies. Local manufacturing capabilities are improving, expanding production capacity and reducing costs, while supportive policies streamline clinical trial approvals and market access. These factors create a fertile environment for rapid adoption of RNA platforms throughout Asia-Pacific, pushing its growth trajectory above other regions and positioning it as a major emerging hub for RNA innovation.
The RNA-based therapeutics and vaccines market is competitive, led by key players like Moderna, Pfizer-BioNTech, BioNTech, CureVac, and Translate Bio. Competition focuses on innovation, clinical efficacy, regulatory approvals, delivery technologies, and pipeline expansion, driving rapid adoption, broader therapeutic applications, and growth across oncology, infectious, and genetic disease markets.
Key Industry Developments:
The global RNA-based therapeutics and vaccines market is projected to be valued at US$ 278.9 Mn in 2026.
Clinical success, rising disease prevalence, strong R&D, technological advances, and regulatory support drive market growth.
The global RNA-based therapeutics and vaccines market is poised to witness a CAGR of 25.5% between 2026 and 2033.
Personalized RNA medicines, cancer vaccines, rare disease therapies, AI integration, and Asia-Pacific market expansion offer opportunities.
Alnylam Pharmaceuticals, Inc., Arbutus Biopharma Corp., Arrowhead Pharmaceuticals, Inc., BioNTech AG, CureVac AG, Dicerna Pharmaceuticals, Inc.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By Indication Type
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author