ID: PMRREP7990| 199 Pages | 8 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

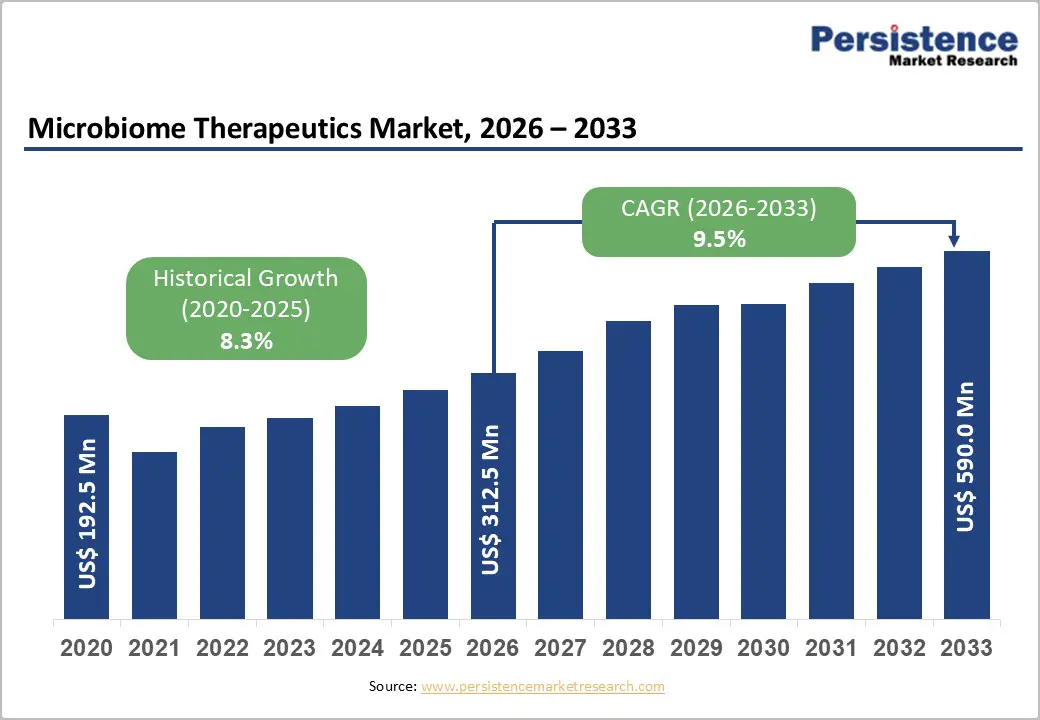
The global microbiome therapeutics market size is likely to be valued at US$312.5 million in 2026 and is projected to reach US$590.0 million by 2033, growing at a CAGR of 9.5% between 2026 and 2033.
The human microbiome, consisting of bacteria, viruses, fungi, and archaea, plays a fundamental role in maintaining health and modulating disease. Advances in microbiome science have revealed its involvement in conditions ranging from inflammatory bowel disease, obesity, and diabetes to allergies, asthma, and certain cancers.
Microbiome therapeutics, including probiotics, live biotherapeutic products (LBP), and fecal microbiota transplantation (FMT) are developed to restore healthy microbial balance, prevent disease progression, and enhance treatment outcomes. With several therapies already approved in specific indications and a robust pipeline of candidates in clinical trials, the field is rapidly transitioning from experimental research to mainstream healthcare, offering significant potential for innovative interventions across multiple therapeutic areas.
| Key Insights | Details |
|---|---|
| Global Microbiome Therapeutics Market Size (2026E) | US$312.5 Million |
| Market Value Forecast (2033F) | US$590.0 Million |
| Projected Growth (CAGR 2026 to 2033) | 9.5% |
| Historical Market Growth (CAGR 2020 to 2025) | 8.3% |
The global microbiome therapeutics market is being fueled by unprecedented advances in human microbiome research. The Human Microbiome Project (HMP) has mapped over 60 million genes, sequenced 700 metagenomes, and established 600 microbial reference genomes, while the MetaHIT catalogue contains 3.3 million non-redundant human microbiome genes, providing foundational insights for translating microbiome science into therapeutics.
Enhanced microbiome sequencing capabilities are accelerating basic research, uncovering microbial diversity, and enabling development of novel therapies. The pipeline of microbiome therapeutics is robust, with over 120 candidates in development targeting gastrointestinal, metabolic, immune, and oncology indications, highlighting the market’s growth potential.
In early 2024, Osel filed a new Investigational New Drug (IND) for CBM588 in oncology and initiated a Phase 1 dose escalation trial in advanced kidney cancer at the City of Hope Comprehensive Cancer Center, exemplifying the move from research to clinical translation.
Further drivers include increasing awareness of microbiome-linked health outcomes, expanding applications in chronic and immune-mediated diseases, and rising investment in R&D by biotech and pharmaceutical companies worldwide. These factors collectively position microbiome therapeutics as a transformative frontier in global healthcare innovation.
The global microbiome therapeutics market faces several notable restraints, despite a promising pipeline. A major challenge is regulatory ambiguity; safety frameworks and approval pathways for live biotherapeutic products (LBPs) are still underdeveloped.
Analytical challenges also pose hurdles, as consistent testing for viability, purity, and potency is difficult without standardized assays. High variability in individual microbiomes complicates clinical trial design and makes translating animal model results uncertain.
Safety concerns, such as unintended colonization, gene transfer, or pathogen transmission in therapies like fecal microbiota transplants, add ethical and long-term monitoring challenges. Combined, these scientific, regulatory, and manufacturing obstacles slow commercialization and create significant barriers to the widespread adoption of microbiome therapeutics.
The global microbiome therapeutics market is witnessing significant opportunities, propelled by increasing venture funding, advanced research, and growing clinical applications. In March 2022, a leading European microbiome biotech secured £50 million ($67 million) in Series B financing, marking the largest microbiome-related funding round in Europe to date.
In October 2022, a Danish microbiome research company received €10 million from Seventure Partners’ Health for Life II, highlighting investor confidence in microbiome science as a transformative frontier for nutrition, disease prevention, and therapy development.
In March 2023, German biotech mbiomics raised €13 million in a Series A round led by MIG Capital to advance precision microbiome therapeutics, leveraging proprietary profiling and computational techniques to optimize microbial consortia and clinical trial strategies.
Most recently, in September 2025, Metagen Therapeutics completed a ¥2.32 billion (≈$15M) Series B financing, supporting the development of an oral fecal microbiota transplantation therapy for ulcerative colitis while expanding organizational capabilities. These investments reflect the global recognition of microbiome science as a next-generation therapeutic platform, opening pathways for innovative products, improved patient outcomes, and accelerated commercialization in both established and emerging markets.
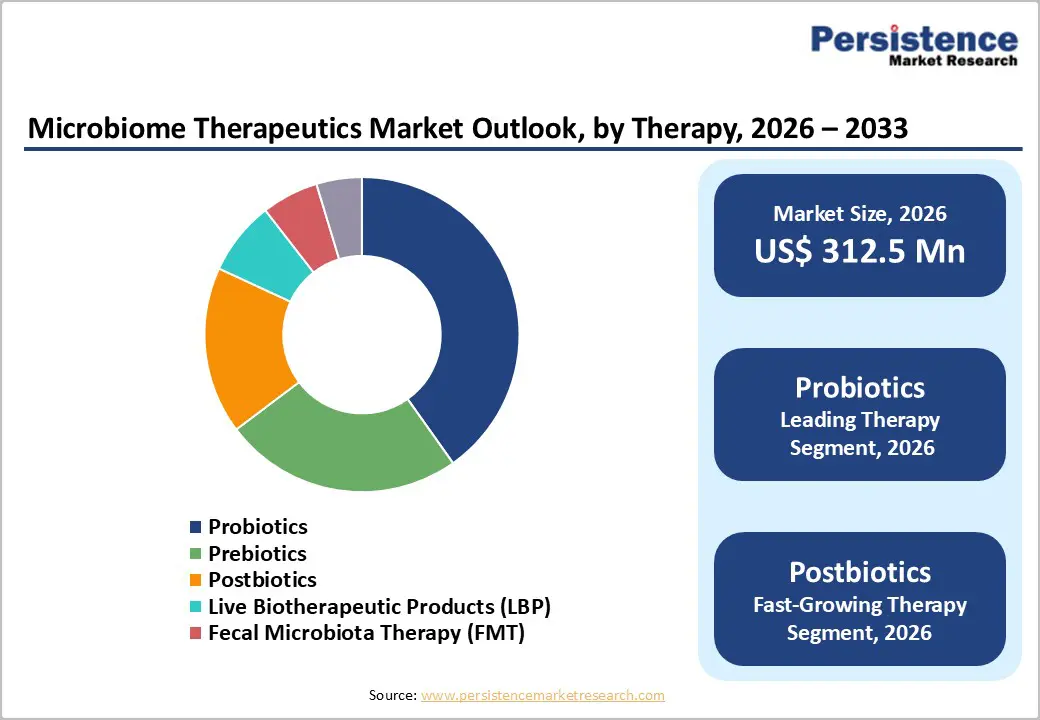
Probiotics are expected to capture a leading 31.8% share of the global microbiome therapeutics market by 2026. Their widespread use in improving gut microbiota balance, supporting immune function, and preventing dysbiosis across multiple indications has driven strong clinical adoption. The growing body of research validating probiotics’ efficacy in gastrointestinal, metabolic, and immune-related disorders further strengthens their dominance. In addition, rising consumer awareness and preference for safe, non-invasive, and natural therapeutic options continue to support the expansion of probiotics in both clinical and over-the-counter settings.
Gastrointestinal disorders are projected to dominate the microbiome therapeutics market in 2026, with a 36.8% share. The increasing prevalence of conditions such as inflammatory bowel disease, irritable bowel syndrome, C. difficile infections, and antibiotic-induced dysbiosis has created strong demand for microbiome-targeted therapies.
Advancements in microbiome science have enabled precise interventions that restore microbial balance, improve patient outcomes, and reduce hospitalization. Growing clinical trial activity and research investments focusing on gut microbiome modulation further reinforce the dominance of gastrointestinal applications in the global microbiome therapeutics landscape.
The oral route is projected to dominate the global microbiome therapeutics market in 2026, capturing nearly 42.2% of the total share. Oral administration offers convenience, non-invasiveness, and high patient compliance, making it ideal for chronic and preventive therapies. It facilitates targeted delivery of probiotics, live biotherapeutics, and microbial consortia to the gut. Regulatory approvals and formulation advancements have enhanced the stability and efficacy of orally delivered microbiome therapies. Combined with widespread physician and consumer acceptance, these factors make oral administration the leading choice for microbiome therapeutics across multiple indications.
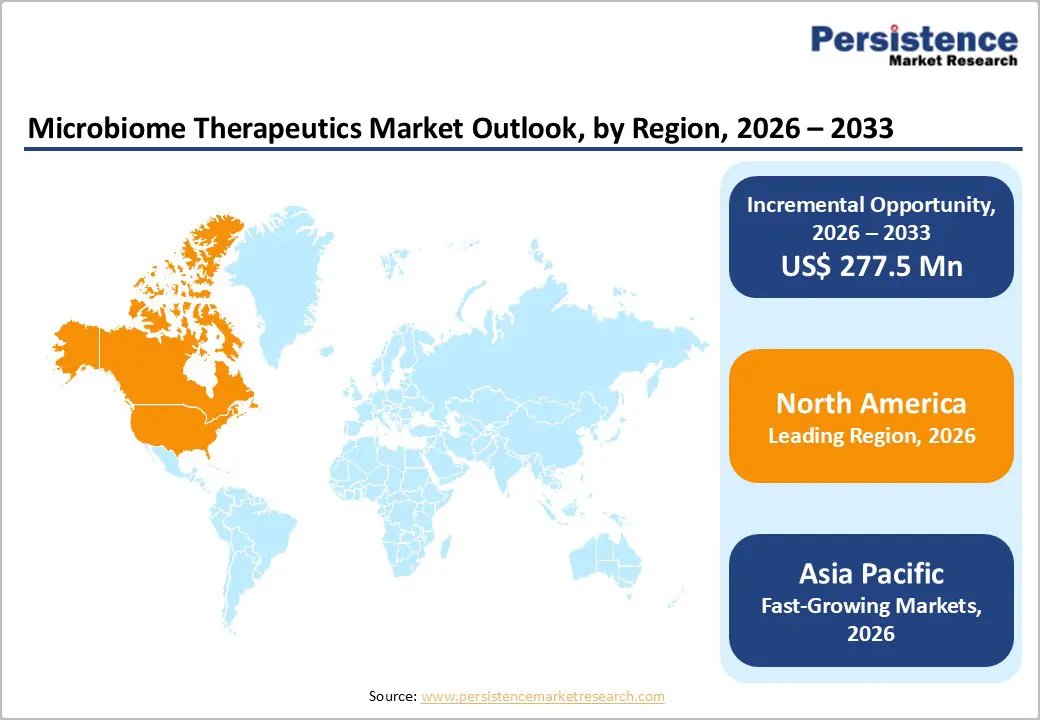
By 2026, North America is projected to account for 43.6% of the global microbiome therapeutics market, supported by a mature innovation landscape, strong investment flows, and rapid clinical translation of microbiome science. The region benefits from one of the world’s most advanced biotech ecosystems, where academic centers, research hospitals, and early-stage biotech firms actively collaborate to accelerate microbiome-based drug discovery.
In October 2025, a U.S.-based microbiome therapeutics company secured $5.22 million from BVM Capital and NOLA Angel Network, reflecting sustained investor confidence despite a competitive environment of 1,574 active players, including 273 funded and 205 exited companies. This dense innovation pipeline drives continuous breakthroughs, positioning North America as a frontrunner in next-generation live biotherapeutics (LBP) and engineered microbial therapies.
Regulatory clarity from the Food and Drug Administration (FDA), including guidance on LBPs, supports faster product advancement, while the rising prevalence of gastrointestinal, metabolic, and immune-mediated disorders expands clinical need. The region also benefits from strong adoption of precision medicine, advanced sequencing technologies, and well-established clinical trial networks capable of running complex microbiome studies. Together, these factors create a robust, opportunity-rich environment that propels North America’s leadership in microbiome therapeutics.
By 2026, Europe is projected to hold 24.4% of the global microbiome therapeutics market, fueled by robust clinical innovation, strategic collaborations, and targeted investments in microbiome-based therapies. The region benefits from a strong pipeline of microbiome ecosystem therapies, particularly in oncology and hematologic conditions.
In July 2023, a leading French biotechnology company specializing in Microbiome Ecosystem Therapies™ (MET) joined the Microbiome Therapeutics Innovation Group (MTIG), a coalition advancing FDA-approved microbiome therapeutics to improve clinical outcomes and reduce healthcare costs. This collaboration was further strengthened in September 2024, when MTIG partnered with the European Microbiome Innovation for Health (EMIH) association to harmonize regulatory frameworks and accelerate microbiome drug development across Europe.
In June 2025, the One Health World Microbiome Partnership Summit in Paris promoted integration of microbiome science into public health, agriculture, and environmental policies under a One Health approach. By July 2025, the same French company secured €37.5 million in financing from the European Investment Bank to advance late-stage clinical trials of microbiome therapies such as Xervyteg® and MaaT033 for hematologic cancers. Combined, strong funding, regulatory harmonization, and high-profile scientific collaborations are driving Europe’s rapid adoption and leadership in microbiome therapeutics.
The Asia Pacific microbiome therapeutics market is projected to grow rapidly at a CAGR of 12.0%, driven by the rising prevalence of metabolic, gastrointestinal, and allergic disorders across the region. Rapid urbanization, changing lifestyles, and increasing incidence of chronic diseases are fueling demand for microbiome-based interventions.
Countries such as China, Japan, and South Korea have emerged as prominent biotech hubs, with strong government support for research, healthcare innovation, and manufacturing infrastructure. Expanding clinical trial activity is accelerating product development, while regulatory modernization, including harmonized frameworks for novel biologics, is facilitating faster approvals and commercialization of microbiome therapeutics.
Notably, in 2024-2025, several microbiome-focused clinical trials were initiated in the region, targeting gastrointestinal disorders, metabolic syndrome, and immune-related conditions, highlighting the growing investment in research and development. In addition, regional collaborations between biotech companies and academic institutions are promoting knowledge sharing, technology transfer, and pipeline expansion.
Combined, these factors are positioning the Asia Pacific as a fast-growing and strategically important region for microbiome therapeutics, with opportunities for both local and multinational companies to introduce innovative therapies to address unmet medical needs.
The global microbiome therapeutics landscape is highly competitive, featuring over 1,500 active companies, including early-stage biotech and established pharma. Key players focus on probiotics, fecal microbiota transplants, and targeted microbial consortia, driving innovation through strategic collaborations, venture funding, and clinical pipeline expansion across gastrointestinal, metabolic, and immune-related indications.
The global microbiome therapeutics market is projected to be valued at US$312.5 Million in 2026.
The understanding of the human microbiome, high disease prevalence, and increasing investment in microbiome-based therapies are driving market growth.
The global market is projected to grow at a CAGR of 9.5% between 2026 and 2033.
Expanding clinical pipelines, rising adoption in emerging markets, and novel microbiome-based therapeutics development present major growth opportunities.
Major players in the global are Microbiome Therapeutics Innovation Group, SERES Therapeutics, Vedanta Biosciences, Inc., Enterome, Ferring, Evotec AG and others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Therapy
By Application
By Route of Administration
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author