ID: PMRREP10094| 190 Pages | 28 Jan 2025 | Format: PDF, Excel, PPT* | Healthcare

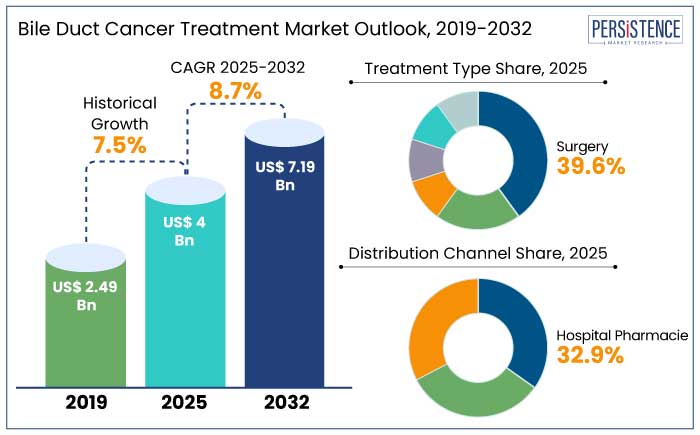
The global bile duct cancer treatment market is projected to witness a CAGR of 8.7% during the forecast period from 2025 to 2032. It is anticipated to increase from US$ 4 Bn recorded in 2025 to a steady US$ 7.19 Bn by 2032.
As cholangiocarcinoma becomes more prevalent and people become increasingly aware of the condition, demand for bile duct cancer treatments is developing. Limited treatment options have spurred developments in diagnostics and therapies, including targeted immunotherapies and kinase inhibitors. For example,
Cutting-edge diagnostic instruments such as liquid biopsies and imaging technologies are transforming early detection and precision medicine. Rising investments in precision oncology and cancer research promise better patient outcomes.
Key Highlights of the Market
|
Market Attributes |
Key Insights |
|
Bile Duct Cancer Treatment Market Size (2025E) |
US$ 4 Bn |
|
Projected Market Value (2032F) |
US$ 7.19 Bn |
|
Global Market Growth Rate (CAGR 2025 to 2032) |
8.7% |
|
Historical Market Growth Rate (CAGR 2019 to 2023) |
7.5% |
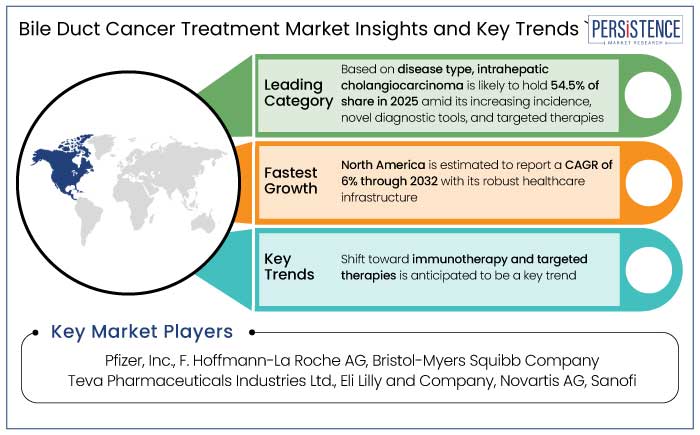
North America is set to hold a 36.8% market share in 2025, making it the largest region in the world for bile duct cancer treatment. The region is estimated to record a CAGR of 6% through 2032.
Risks associated with lifestyle choices, such as obesity, diabetes, chronic liver disease, and an aging population, are the main drivers of this rise. Innovative biopharmaceutical businesses, robust research and development skills, and sophisticated healthcare systems all support the market's growth.
Early screening and awareness initiatives also increase the number of diagnoses, and the robust biopharmaceutical industry in the area spurs innovation. North America's supremacy is ascribed to early diagnosis, treatment understanding, and reimbursement regulations, with 10,000 new cases reported each year. Research and development for novel medicines is another area of concentration for key industry participants.
In 2025, Asia Pacific is estimated to account for 26% of the global market share, with a forecasted CAGR of 7.5% from 2025 to 2032. For example,
Growth of telemedicine services, which doubled during the worldwide pandemic and leveled out in 2023, is one development. Companies such as Bain & Company have emphasized the increase in consumer interaction and how technology is altering healthcare.
The ‘Health at a Glance: Asia/Pacific 2024’ study from the OECD emphasizes the region's developments in telehealth, high consumer expectations, and an aging population as drivers of universal health care.
The most prevalent kind of cholangiocarcinoma, making up a sizable percentage of cases, is intrahepatic cholangiocarcinoma (ICC). The ICC is set to account for 54.5% of share in 2025 owing to its early indication where surgical resection is more feasible.
The best course of therapy is still surgery, particularly for liver-localized instances. Its popularity has been aided by developments in targeted treatments and knowledge of the molecular properties of ICC.
Pemigatinib and ivosidenib are examples of individualized therapy techniques that have been made possible by the discovery of genetic alterations and biomarkers. Demand for ICC therapy grows as its incidence increases, solidifying its position as the industry leader in bile duct cancer treatment.
Hospital pharmacies are the leading providers of bile duct cancer treatment due to their experience with complicated illnesses and the specific care required for treatment. The segment is anticipated to account for 32.9% of the global market share in 2025.
Novel options such as immunotherapies, targeted therapies, and chemotherapy drugs are frequently used throughout treatment. These are usually provided in a hospital. Hospitals are the recommended setting for treating people with bile duct cancer since these treatments need specialized tools and medical personnel.
Hospital pharmacists ensure that experimental or chemotherapeutic medicines are delivered on schedule and that patients are monitored. Hospitals also house an interdisciplinary team of nurses, pharmacists, and oncologists assuring proper administration and dose adjustments.
The demand for bile duct cancer treatments is rising rapidly because of the surging prevalence of this uncommon but deadly kind of cancer. It is being caused by aging populations, obesity, and chronic liver illnesses.
The most common kind of bile duct cancer is cholangiocarcinoma. The market is marked by an increasing need for innovative treatment alternatives, especially for unique and incurable stages.
With continuous research aimed at increasing efficacy and survival rates, treatment options include immunotherapies, targeted treatments, chemotherapy, and surgery. In 2025, surgeries associated with cancer are set to account for 39.6% of the global market share.
Patients with certain genetic mutations are increasingly receiving targeted medicines, including pemigatinib and ivosidenib, which have shown encouraging results in terms of bettering patient outcomes. Checkpoint inhibitors and other immunotherapies are becoming a leading therapy trend for advanced-stage cholangiocarcinoma.
The global bile duct cancer treatment market recorded a decent CAGR of 7.5% in the historical period from 2019 to 2023. Due to improved awareness, novel treatment choices, and breakthroughs in diagnosis, the market for bile duct cancer treatments has grown steadily in recent years.
Chemotherapy has traditionally been the main treatment for later stages, however new developments in immunotherapies and targeted medicines have completely changed the therapeutic options. The market is set to continue rising as the prevalence of bile duct cancer surges worldwide, especially due to chronic liver diseases and an aging population.
Research on genetic markers and biomarkers, as well as the easy availability of sophisticated treatment choices, hold promise for improving patient outcomes. These are anticipated to augment growth of treatment modalities and market value.
Potential of Immunotherapy Enhances Long-term Outcomes for Cancer Treatment
Immunotherapy, which targets and kills cancer cells, is becoming immensely popular in the treatment of bile duct cancer, especially extrahepatic cholangiocarcinoma. This strategy is a leading development in the bile duct cancer therapy market, propelled by continuous investigation into different immunotherapeutic approaches. For instance,
According to the results of the research, individuals with advanced bile duct cancer may benefit from immunotherapy's long-lasting effects and enhanced survival rates.
Rising Cancer Awareness to Bolster Efficiency of Screening Rates
Growing knowledge of bile duct cancer's causes, risk factors, and early identification is driving the market's growth. For high-risk groups in particular, routine screening is essential.
Physicians can now recognize warning signs and symptoms because they have a better grasp of the illness. Most nations have seen an increase in screening rates as a result, which has resulted in more cases being identified at an earlier stage and more effective treatment options.
Less intrusive techniques are frequently needed for early identification, which increases demand for bile duct cancer services and treatments. Cancer societies and support groups can increase screening rates and early detection by educating the public. For instance,
High Treatment Expenses May Limit Adoption in Emerging Regions
The substantial difference in cancer treatment expenses between developed and underdeveloped nations is brought to light by the American Society of Clinical Oncology.
International treatment possibilities have been impacted by the severe travel restrictions imposed by the worldwide epidemic. It is predicted that these limitations will remain in place until the end of 2022, with possible modifications to travel laws planned in 2023. Demand for cancer treatments is anticipated to affect the healthcare industry with new international travel regulations and evolving medical trends.
Research Collaborations to Give Rise to Novel Therapeutic Approaches
Innovative therapeutic techniques, such as immunotherapy and targeted medicines, are anticipated to help emerging firms in the bile duct cancer treatment market stand out from the competition. These cutting-edge therapies provide individualized, efficient care for unmet medical requirements. New businesses should spend money on clinical studies to increase their legitimacy and regulatory clearances.
To share information and have access to complementary skills, collaboration between academic institutes and industry partners is essential. Such collaborations will likely expedite research and development activities, allowing new players to more effectively provide novel therapies and bolster the market's projected growth for cancer treatments.
Governments across the Globe to Support Innovation in Cancer Treatments
Combination therapies are becoming increasingly significant in the treatment of bile duct cancer as these enable physicians to approach the illness from several perspectives, overcome resistance, and outperform single-agent treatments. Immunotherapy and chemotherapy in combination with radiation treatment are two examples. According to clinical research, resection rates and long-term results may be enhanced by combining chemotherapy and radiation treatment before surgery. For example,
Global behemoths like Roche, Bristol-Myers Squibb, and Eli Lilly are at the forefront of targeted medicines and immunotherapies in the bile duct cancer therapy industry. It is made up of both established and up-and-coming companies. Players like AstraZeneca, Incyte, and Mirati Therapeutics are investigating novel treatments and customized medicine.
To speed up medication development, new participants in the market are forming strategic alliances with existing pharmaceutical corporations and university research organizations. Gaining a competitive edge requires clinical trials, and future tactics will probably be influenced by the focus on genetic testing and tailored care. The market is anticipated to surge quickly due to new therapy methods and innovation.
?Recent Industry Developments
The market size is set to reach US$ 7.19 Bn by 2032.
In November 2024, the FDA approved Ziihera for treating advanced and metastatic bile duct cancer.
In 2025, North America is set to attain a market share of 36.8%.
In 2025, the market is estimated to be valued at US$ 4 Bn.
Pfizer, Inc. and F. Hoffmann-La Roche AG are considered the leading players.
|
Attributes |
Details |
|
Forecast Period |
2025 to 2032 |
|
Historical Data Available for |
2019 to 2023 |
|
Market Analysis |
US$ Billion for Value |
|
Key Countries Covered |
|
|
Key Market Segments Covered |
|
|
Key Companies Profiled in the Report |
|
|
Report Coverage |
|
|
Customization & Pricing |
Available upon request |
By Treatment Type
By Disease Type
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author