ID: PMRREP18343| 199 Pages | 23 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

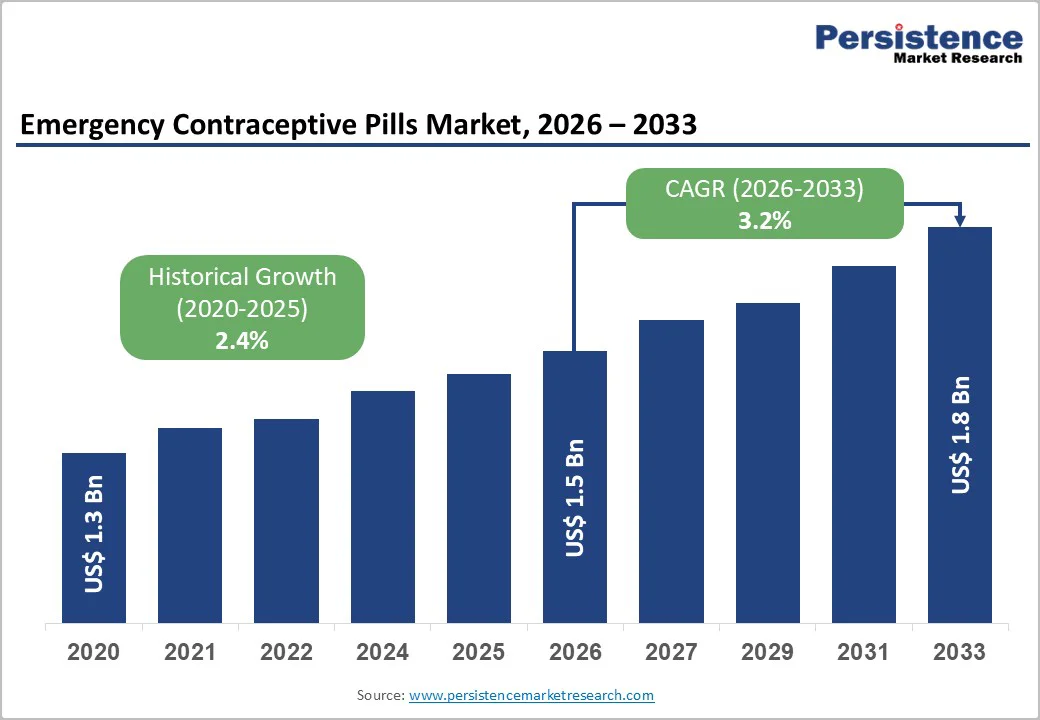
The global emergency contraceptive pills market size is estimated to grow from US$ 1.5 billion in 2026 to US$ 1.8 billion by 2033. The market is projected to record a CAGR of 3.2% from 2026 to 2033.
Rising awareness of reproductive rights, broader over- the-counter (OTC) availability, and supportive public health policies are sustaining steady market expansion.
Global health agencies recognize levonorgestrel, ulipristal acetate and certain combined oral contraceptive regimens as effective emergency methods, and more than 60-70 countries now include at least one emergency contraceptive pill (ECP) on essential medicines or OTC lists, which continues to normalize use and strengthen demand.
| Key Insights | Details |
|---|---|
| Emergency Contraceptive Pills Market Size (2026E) | US$ 1.5 Bn |
| Market Value Forecast (2033F) | US$ 1.8 Bn |
| Projected Growth (CAGR 2026 to 2033) | 3.2% |
| Historical Market Growth (CAGR 2020 to 2025) | 2.4% |
Growing awareness of reproductive health rights and supportive policy actions is a central force driving the emergency contraceptive pills (ECP) market. Over the past decade, governments, NGOs, and healthcare agencies have expanded public education on modern contraception, emphasizing the safety and effectiveness of levonorgestrel, ulipristal acetate, and combined emergency regimens.
The World Health Organization’s endorsement of these methods has strengthened confidence among clinicians and users, prompting inclusion of ECPs in national family-planning protocols.
Regulatory reviews across multiple regions show that roughly 43% of evaluated countries now have structured pathways for transferring selected prescription-only contraceptives to over-the-counter (OTC) status. This reform directly benefits emergency contraception by reducing barriers to access.
Adolescents, young adults, and women with limited access to healthcare particularly benefit from this shift, as OTC availability eliminates the need for time-sensitive consultations. Parallel efforts by professional bodies and community-health programs have improved understanding of proper use, safety parameters, and timing, reducing stigma and misinformation.
As awareness rises and health systems prioritize unintended pregnancy prevention, demand for accessible, pharmacy-based ECP options continues to strengthen across both developed and emerging markets.
Despite growing acceptance, regulatory inconsistencies remain a major limiting factor for the emergency contraceptive pills market. Cross-country assessments reveal that fewer than half of national regulatory authorities have formal procedures for switching contraceptives from prescription status to OTC, a necessary step for broader access.
In several regions, ECPs remain prescription-only, age-restricted, or subject to special pharmacist-authorization rules, creating delays that reduce their effectiveness window. These hurdles disproportionately affect young women and those in rural or low-resource settings who may be unable to secure quick consultations.
Furthermore, emergency contraceptives are inconsistently listed on public reimbursement programs or essential-medicine formularies, resulting in higher out-of-pocket expenses, particularly where imported brand-name options dominate pharmacy shelves.
Variations in marketing approvals and import regulations can also slow the entry of affordable generics. Together, these factors suppress uptake, limit distribution through retail channels, and contribute to persistent disparities in access across countries and demographic groups.
A major opportunity for market expansion lies in widening OTC availability and strengthening pharmacist-driven access pathways. Levonorgestrel-based ECPs are registered in over 100 countries, with a steadily increasing proportion permitting non-prescription purchase. Ulipristal acetate is marketed in roughly 65 countries, and close to 40 allow OTC or pharmacist-supplied access.
Regions that have embraced these models, such as much of Europe, North America, and parts of Latin America, report higher awareness, smoother purchasing experiences, and significantly greater uptake. Retail pharmacies have become central distribution hubs for leading brands, including Plan B One-Step®, NorLevo®, ellaOne®, and multiple generic levonorgestrel formulations.
As more regulators adopt OTC switches, permit pharmacist prescribing, or enable protocol-based supply, manufacturers gain opportunities to establish partnerships with large pharmacy chains, expand educational outreach, and introduce discreet packaging and private counseling areas.
These changes not only improve consumer comfort but also support timely access, which is critical for clinical effectiveness. Expanding digital pharmacy integration, enabling click-and-collect or same-day delivery, further enhances growth potential, especially in urban and emerging markets.
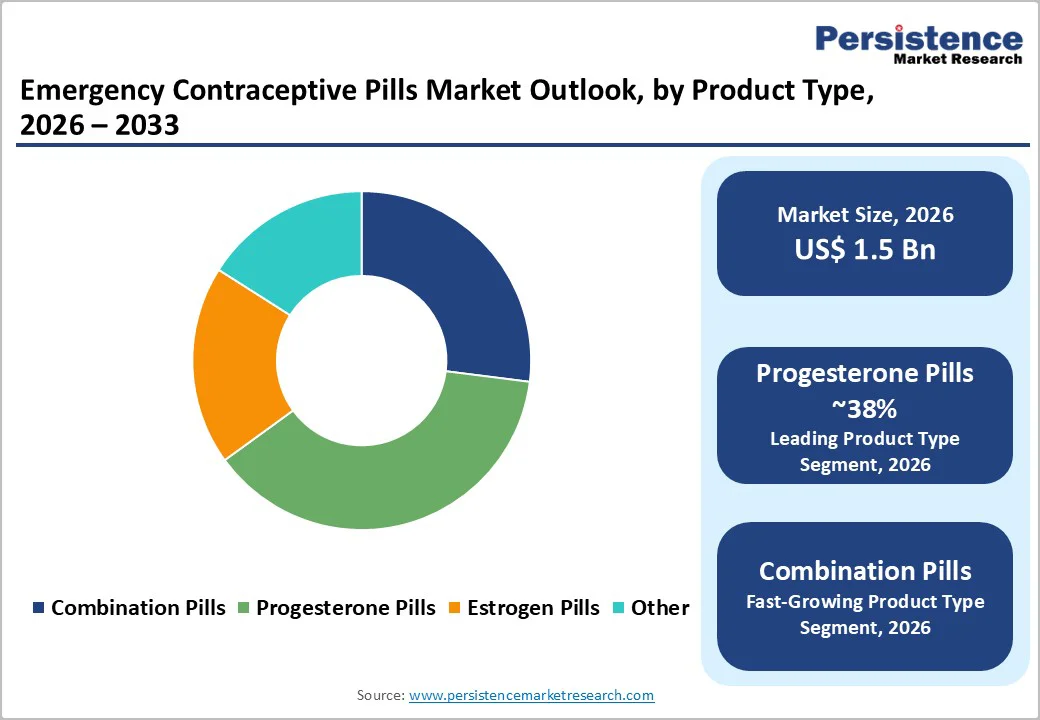
Progesterone-only pills, mainly levonorgestrel 1.5 mg formulations, are projected to hold the largest share of the emergency contraceptive market by 2025, reaching roughly 38%. Their strong position reflects widespread regulatory acceptance, simple single-dose schedules, and broad suitability for users of different ages and health backgrounds.
International guidelines from bodies such as the WHO and CDC consistently list levonorgestrel as a frontline option because of its reliable safety record and ease of use without medical supervision. Countries that allow OTC access have further strengthened their market lead by placing these products directly in pharmacies and retail stores, making them the most familiar and accessible ECP choice for the public.
Published clinical evidence shows high effectiveness when taken within the recommended 72-hour window, with very few severe side effects, encouraging procurement by national family-planning programs and NGO supply chains. Estrogen-containing and combined-hormone pills remain available in certain markets but tend to serve as supplementary alternatives rather than competing with progesterone-only dominance.
Pharmacies and drug stores remain the primary distribution channel for emergency contraceptive pills, accounting for an estimated 45% of global sales in 2025. Their dominance stems from immediate product availability, extended operating hours, and the familiarity users have with seeking discreet advice from community pharmacists.
Many countries permit direct OTC purchase of levonorgestrel pills at these outlets, making pharmacies the most convenient point of access during the time-sensitive window when ECPs work best. Large retail chains and supermarket-based pharmacies also contribute significantly, stocking leading brands and offering consistent supply through high-traffic locations.
User surveys frequently highlight privacy, quick access, and avoidance of clinical appointments as key reasons for choosing pharmacies over other channels. While online platforms and tele-pharmacy providers are gaining ground, especially in urban areas with strong digital adoption, the majority of sales still flow through brick-and-mortar pharmacies because of regulatory comfort and the availability of pharmacist counseling.
Hospital pharmacies contribute smaller but steady volumes, mainly linked to post-assault care and inpatient needs.
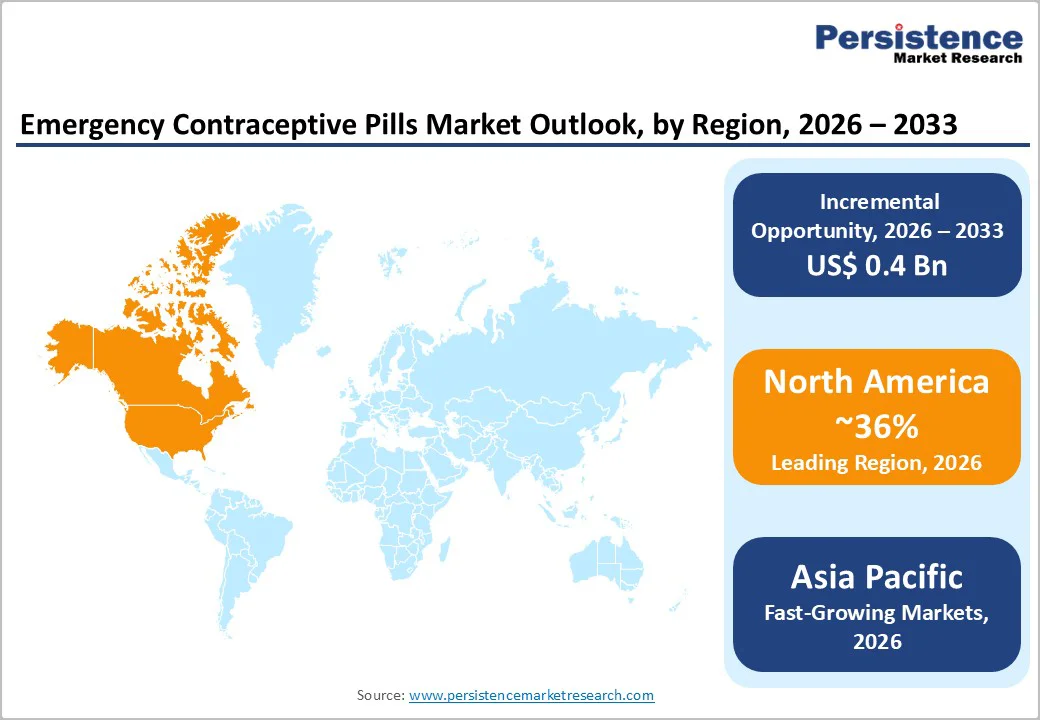
North America, led by the U.S., is the largest regional market, accounting for an estimated 36% share of global emergency contraceptive pill revenues in 2026. The U.S. Food and Drug Administration (FDA) has progressively liberalized access, allowing levonorgestrel ECPs, such as Plan B One-Step® and generics, to be sold OTC without age restrictions, thereby normalizing stocking across major pharmacy chains and supermarkets.
National guidelines from organizations such as the American College of Obstetricians and Gynecologists (ACOG) and the Society of Family Planning explicitly recommend advance provision and pharmacy-based access, thereby further encouraging utilization.
Canada and parts of Mexico have also adopted pharmacy-access or OTC models under professional protocols. At the same time, strong insurance coverage and public-health campaigns emphasize contraception choice and unintended-pregnancy prevention.
The regional innovation ecosystem includes branded and generic levonorgestrel pills, prescription-only ulipristal acetate, and integration with telemedicine platforms that can issue rapid e-prescriptions, supporting continued growth in both physical and digital channels.
The Asia Pacific region is expected to be the fastest-growing market for emergency contraceptive pills, driven by large reproductive age populations in China, India and ASEAN countries and increasing urbanization.
Policy mapping by the Asia Pacific Contraception Policy Atlas shows significant variation. Still, many governments are gradually expanding access to modern contraception, including ECPs, through public sector programs and social-marketing initiatives.
In India, levonorgestrel emergency pills are widely available through pharmacies and public health outlets, and state-supported campaigns have promoted awareness among adolescents and newly married couples, thereby raising demand for low-cost generic brands.
In China, national family-planning services and urban pharmacies provide both prescription and OTC ECPs, while emerging e-commerce platforms cater to privacy-conscious consumers. Several ASEAN markets, including Thailand and the Philippines, are experiencing gradual regulatory relaxation and stronger NGO engagement, though access remains uneven in more conservative settings.
Local manufacturers and regional generics firms are playing an increasingly important role in supplying affordable levonorgestrel products, leveraging cost advantages and expanding distribution networks into tier-2 and rural areas.
The emergency contraceptive pills market is moderately consolidated, with a mix of multinational pharmaceutical companies and regional generics manufacturers.
Global leaders such as Teva Pharmaceutical Industries Ltd., Bayer AG, HRA Pharma, Pfizer Inc., Mankind Pharma Ltd., Lupin Limited, and Richter Gedeon Nyrt offer branded and generic levonorgestrel or ulipristal products tailored to national regulations and price points.
Key differentiators include time window of effectiveness (e.g., ulipristal’s 120-hour use), OTC versus prescription status, brand recognition, and valued packaging or counseling tools. Companies are focusing on portfolio expansion into low-dose and combined formulations, supply-chain optimization with large pharmacy chains, and partnerships with NGOs and digital platforms to reach underserved segments.
The global contraceptive pills market is projected to be valued at US$ 1.5 Bn in 2026.
Growing awareness, OTC access, strong clinical evidence, and convenient single-dose options continue to drive global emergency contraceptive pill demand.
The global contraceptive pills market is poised to witness a CAGR of 3.2% between 2026 and 2033.
Major opportunities include broader OTC availability, tele-pharmacy expansion, and affordable generics gaining traction in rapidly growing emerging markets.
Key companies include Teva Pharmaceutical Industries Ltd., Bayer AG, HRA Pharma, Piramal Enterprises Limited, Mankind Pharma Ltd., Richter Gedeon Nyrt, and Lupin Limited.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn and Volume (if Available) |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Sales Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author