ID: PMRREP4788| 192 Pages | 2 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

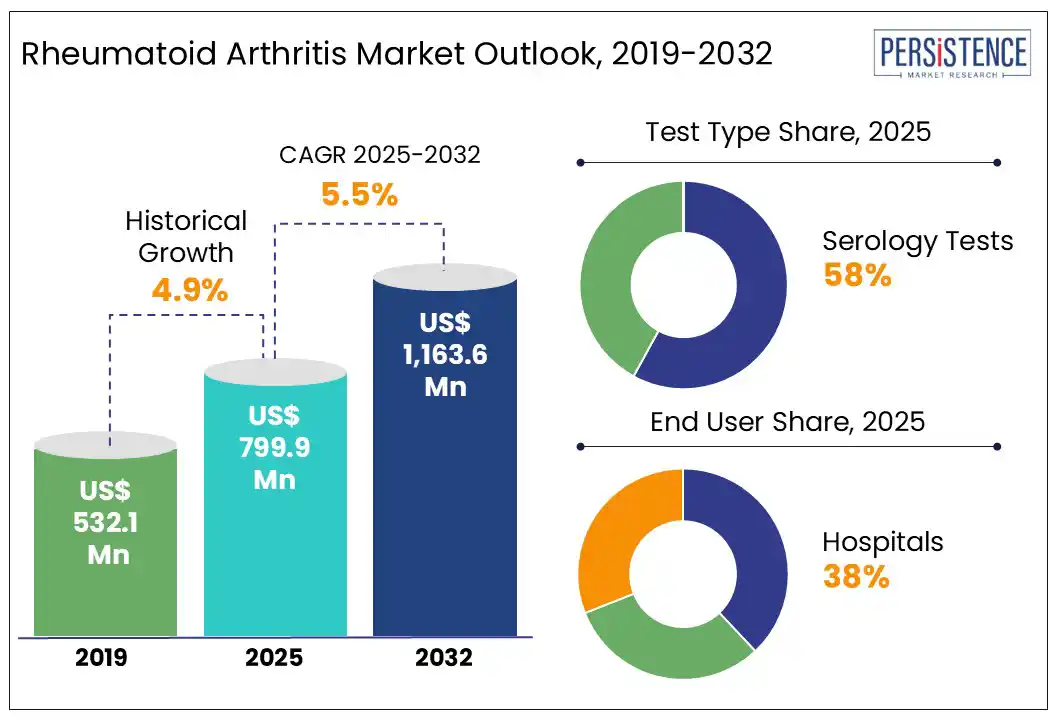
The global rheumatoid arthritis market size is likely to be valued at US$ 799.9 Mn in 2025 and is expected to reach at US$ 1,163.6 Mn by 2032, growing at a CAGR of 5.5% from 2025 to 2032.
According to Persistence Market Research report, the rheumatoid arthritis service market is evolving rapidly, driven by the need for comprehensive disease management beyond drug therapy. With rising RA prevalence and the chronic nature of the disease, demand for diagnostic services, infusion therapy, physiotherapy, telehealth, and long-term patient monitoring is surging. Service providers are focusing on integrated care models that combine in-person consultations, virtual care, and home-based services. North America leads the market due to advanced rheumatology clinics and digital health adoption, while Asia-Pacific is emerging with growing investments in outpatient and rehabilitation services. The shift toward value-based care, personalized support programs, and remote disease tracking is transforming RA services into a holistic ecosystem supporting patient outcomes, adherence, and quality of life.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Rheumatoid Arthritis Market Size (2025E) |
US$ 799.9 Mn |
|
Market Value Forecast (2032F) |
US$ 1,163.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
4.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.5% |
Anti-CCP antibody testing continues to solidify its role as the gold standard in rheumatoid arthritis (RA) diagnostics due to its unmatched specificity and predictive value. As of 2024, clinical guidelines globally are recommending anti-CCP testing alongside rheumatoid factor (RF) for early and accurate RA detection, particularly in patients with undifferentiated arthritis. Recent studies highlight its ability to detect RA up to a decade before symptoms appear, making it crucial for preclinical diagnosis. Moreover, growing adoption of multiplex autoantibody panels and next-generation immunoassay platforms has enhanced test efficiency, reducing turnaround time and improving accessibility. Diagnostic labs and hospitals are increasingly utilizing automated CCP testing kits approved by regulatory bodies such as the FDA and CE. In addition, ongoing research is uncovering the role of anti-CCP in treatment response prediction, further integrating it into precision medicine frameworks. With the increasing demand for early, accurate, and personalized RA care, anti-CCP testing is expected to see robust growth globally.
Diagnostic Overlap with other disorders poses a significant challenge in the rheumatoid arthritis (RA) diagnostic market. RA shares overlapping clinical manifestations, such as joint pain, stiffness, and inflammation, with other autoimmune diseases such as systemic lupus erythematosus (SLE) and psoriatic arthritis (PsA). Moreover, common biomarkers such as rheumatoid factor (RF) and antinuclear antibodies (ANA) may test positive in multiple conditions, leading to diagnostic ambiguity. This overlap often results in misclassification, delayed diagnosis, or inappropriate treatment initiation. The heterogeneity in patient presentations further complicates differential diagnosis, especially in early-stage RA. Consequently, physicians require a combination of serological tests, imaging, and clinical judgment, which increases diagnostic complexity and costs. This restraint underscores the urgent need for more disease-specific biomarkers and integrated diagnostic algorithms.
Clinical Decision Support Systems (CDSS) for rheumatoid arthritis (RA) diagnosis present a transformative opportunity in early disease detection and referral optimization. These intelligent platforms integrate patient-reported symptoms, lab markers such as rheumatoid factor (RF) and anti-CCP antibodies, and imaging data (e.g., X-ray or ultrasound findings) into a unified decision-making algorithm. Designed for primary care settings, CDSS tools alert general practitioners to early RA indicators and suggest timely specialist referrals before irreversible joint damage occurs. By leveraging real-time analytics, machine learning, and historical patient data, these systems minimize diagnostic delays and improve patient outcomes. The integration of CDSS into electronic health records further enhances diagnostic accuracy, reduces misclassification, and supports evidence-based care pathways for managing complex autoimmune conditions such as RA.
Serology account for the highest share in the test type segment of the rheumatoid arthritis (RA) market due to their critical role in early and accurate diagnosis. These tests, including rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibody detection, are widely used as primary diagnostic tools to confirm autoimmune activity. Their high specificity, rapid turnaround, cost-effectiveness, and compatibility with routine laboratory workflows make them indispensable in both primary and specialist care. Additionally, serology tests are often the first line of investigation when patients present with joint pain, enabling early intervention and improved disease management. Their widespread availability and established clinical value have led to consistent adoption, securing their dominance in the RA diagnostic landscape.
Hospitals dominate the end-use segment due to their integrated infrastructure, multidisciplinary care, and access to advanced biologics and infusion therapies. RA management often requires continuous monitoring, imaging, lab diagnostics, and specialist consultations all centralized in hospital settings. Moreover, biologic therapies such as TNF inhibitors and JAK inhibitors are frequently administered in infusion centers located within hospitals, reinforcing patient dependence on these facilities. In severe RA cases, hospitalization for joint surgeries or complications becomes essential, further increasing service utilization. Additionally, reimbursement policies in many countries favor hospital-based care for chronic conditions, making hospitals, a primary hub for both acute and long-term RA treatment. This convergence of advanced care and institutional support drives their leading market share.
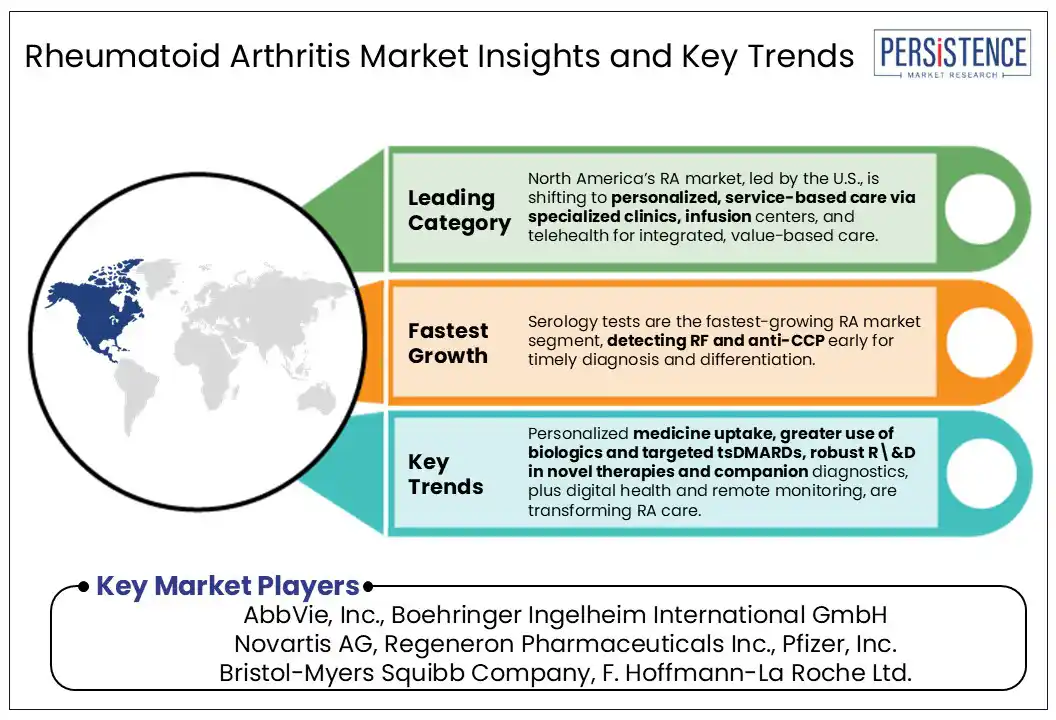
In North America, particularly the U.S., the rheumatoid arthritis (RA) market is witnessing a shift toward personalized, service-based care models. The rise of specialized rheumatology clinics, infusion centers, and telehealth platforms has expanded access to continuous disease management services. U.S. healthcare systems emphasize value-based care, driving integrated service delivery that combines diagnostics, biologic therapy administration, and patient education under one roof.
Approximately 18 million people globally are affected by rheumatoid arthritis, including around 1.5 million in the United States. The condition is nearly three times more prevalent in women than in men. Moreover, increasing adoption of biosimilars and home-based infusion services reflects a cost-sensitive yet patient-centric trend. The demand for remote monitoring and digital tools, supported by favorable reimbursement and private insurance policies is reshaping how RA services are delivered. These evolving service ecosystems position the U.S. as a frontrunner in transforming RA care into a holistic, service-driven market model.
In Europe, the rheumatoid arthritis (RA) market is witnessing a significant shift toward outpatient specialty clinics and home-based infusion services, reducing dependency on traditional hospital settings. Countries such as Germany, the UK, and France are actively integrating tele-rheumatology services, enabling remote monitoring and virtual consultations. The adoption of biosimilars has surged across Europe due to stringent cost-containment policies and centralized procurement by public health systems. Furthermore, the region emphasizes early diagnosis through national screening programs, especially in Scandinavian countries. Patient registries, such as Sweden’s SRQ, enhance treatment personalization and real-world data collection. Combined with value-based care initiatives and EU-wide reimbursement reforms, Europe’s RA service model is evolving toward decentralized, cost-efficient, and technology-driven management frameworks that improve patient accessibility and outcomes.
In Asia Pacific, the rheumatoid arthritis (RA) market is rapidly evolving, driven by expanding healthcare infrastructure, rising disease awareness, and increasing access to specialty care services in urban centers. As an emerging market, countries such as India, China, and Southeast Asia are experiencing a surge in early diagnosis and treatment, supported by government health programs and digital health platforms that extend rheumatology services to remote areas. Unlike Western regions, a significant portion of care is still delivered through public hospitals and tertiary care centers due to affordability concerns. Additionally, the growing adoption of biosimilars is reshaping treatment paradigms, making advanced therapies more accessible. Medical tourism in countries like Thailand also contributes to RA service market growth in the region.
The rheumatoid arthritis (RA) service-based market is becoming increasingly competitive, with hospitals, specialty clinics, and telehealth providers vying for patient engagement and retention. Major healthcare networks are enhancing service delivery through integrated care pathways, digital platforms, and personalized treatment protocols. Private rheumatology centers are emerging rapidly, offering faster access to biologics and infusion therapies. Meanwhile, tele-rheumatology services are expanding, especially for follow-ups and rural outreach.
The global rheumatoid arthritis market is estimated to increase from US$ 799.9 Mn in 2025 to US$ 1,163.6 Mn in 2032.
The global rheumatoid arthritis market is primarily propelled by the rapidly rising prevalence of the disease, now affecting millions worldwide, as populations age and lifestyle risk factors increase.
The market is projected to record a CAGR of 5.5% during the forecast period from 2025 to 2032.
The rheumatoid arthritis drug market is experiencing growth, with increasing prevalence of arthritis, obesity, and unhealthy lifestyles driving demand.
Serology accounts for the highest share in the test type segment of the rheumatoid arthritis market due to its critical role in early and accurate diagnosis.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Test Type
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author