ID: PMRREP2782| 199 Pages | 16 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

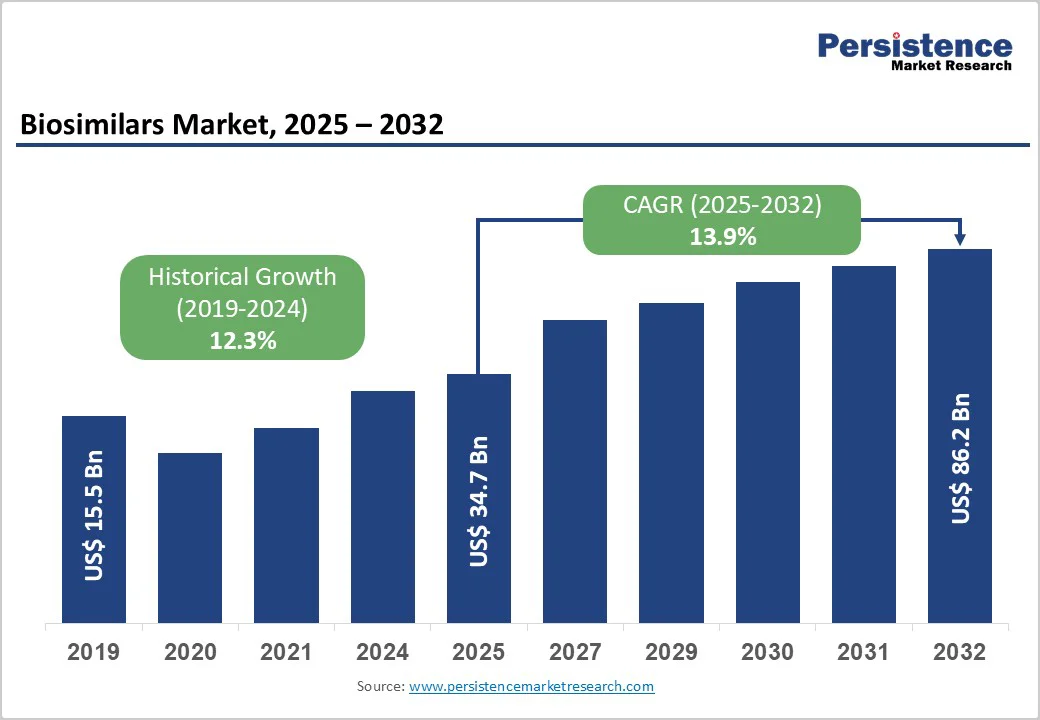
The global biosimilars market is valued at US$34.7 billion in 2025 and is projected to reach US$86.2 billion by 2032, growing at a CAGR of 13.9% from 2025 to 2032.
The global market is expanding steadily, driven by rising chronic disease prevalence, expiring biologics patents, and strong demand for affordable therapies. North America leads due to supportive regulatory pathways, while Asia-Pacific is the fastest-growing region, fueled by expanding healthcare access, higher biosimilar adoption, and growing domestic manufacturing capabilities.
| Key Insights | Details |
|---|---|
|
Global Biosimilars Market Size (2025E) |
US$34.7 Bn |
|
Market Value Forecast (2032F) |
US$86.2 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
13.9% |
|
Historical Market Growth (CAGR 2019 to 2024) |
12.3% |
A major driver of the biosimilars market is the patent expirations of high-value biologics, which unlock large opportunities for cost-effective alternatives. For instance, Humira (adalimumab), once the world’s best-selling drug with peak annual sales over $20 billion, lost exclusivity in the U.S. in 2023, enabling multiple biosimilar launches. Similarly, Stelara (ustekinumab), generating more than $9 billion annually, saw its key U.S. patents expire in 2023–2024, allowing the first interchangeable biosimilar to enter the market in 2025. In total, over 60 major biologics patents are scheduled to expire between 2024 and 2030 in the U.S. and EU, affecting therapies across oncology, immunology, diabetes, and rare diseases.
These experiences open multi-billion-dollar markets to biosimilars, encouraging competition, lowering treatment costs, and accelerating adoption across hospitals, specialty clinics, and pharmacy channels. As more blockbuster biologics face loss of exclusivity, biosimilar penetration continues to rise, driving strong market growth.
Developing a biosimilar typically requires $100–300 million and takes 6–9 years, significantly higher than small-molecule generics. The production involves living cells, and even minor variations in cell culture conditions, pH, or temperature can alter glycosylation patterns, impacting drug safety and efficacy. Manufacturing costs for recombinant proteins average around $300 per gram, with large-scale facilities targeting up to $500 per gram, making production capital-intensive. Additionally, stringent quality control, extensive analytical testing, and compliance with regulatory standards increase both time and costs.
High entry barriers limit smaller companies from entering the market and reduce competitive pressure, slowing adoption. These manufacturing complexities, combined with long development timelines, contribute to limited market penetration in some regions despite strong demand, making cost and technical challenges persistent restraints on biosimilar growth.
As biologics like checkpoint inhibitors (e.g., Keytruda and Opdivo) and anti-TNF agents approach patent expiry, biosimilar manufacturers can tap into massive revenue pools: one analysis estimates that 61% of the upcoming biosimilar opportunity (2024–2028) comes from oncology, while 17% is in immunology. This reflects the sheer scale of high-selling biologics in these areas - for instance, oncology biologics made up 51% of the global cancer-drug market in 2024, largely driven by innovative monoclonal antibodies.
Biosimilars in these categories are already launching at 10–25% discounts relative to originators. Additionally, oncology biosimilars have demonstrated fast uptake. Within five years of launch, they’ve averaged 81% market share, making them highly attractive to manufacturers seeking scale and long-term profitability.
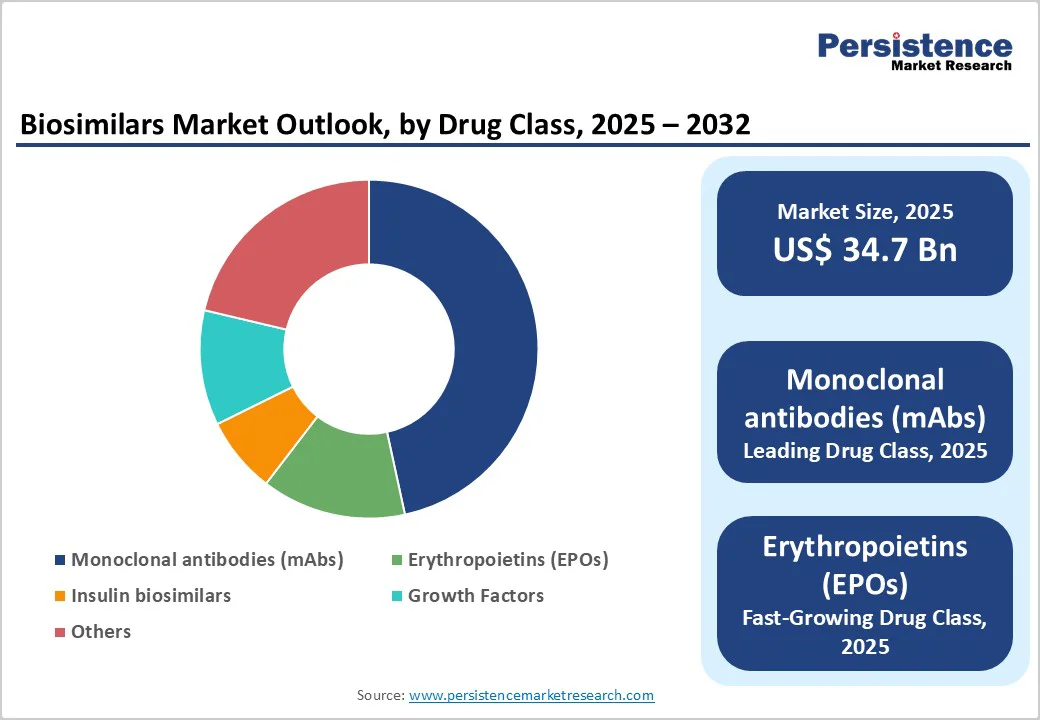
Monoclonal antibodies (mAbs) account for 46.6% of the global market in 2025, driven by their high clinical value in oncology and autoimmune diseases. Currently, over 80 biosimilars targeting 38 reference biologics are in development, many focusing on mAbs such as trastuzumab, rituximab, and bevacizumab, which collectively generated billions in annual sales—trastuzumab alone exceeded $7 billion before biosimilar entry.
High demand for cost-effective alternatives in conditions like breast cancer, rheumatoid arthritis, and inflammatory bowel disease drives adoption. Regulatory pathways in the U.S. and Europe have enabled multiple mAb biosimilars to gain approval, ensuring safety and interchangeability. Additionally, well-established manufacturing processes and predictable clinical outcomes make mAbs attractive for biosimilar developers. Their high revenue potential and clinical significance position mAbs as the leading drug class in the biosimilars market.
Autoimmune disorders dominate the biosimilars market because many top-selling biologics are used to treat chronic inflammatory conditions. Biosimilars have rapidly replaced originator drugs in this segment, with infliximab accounting for 93% and etanercept for 98% of total use in markets like Germany. Conditions such as rheumatoid arthritis, psoriasis, and Crohn’s disease drive strong clinical demand. In the U.S., over 20 million people are estimated to have at least one autoimmune disease, with annual treatment costs exceeding $168 billion. Biosimilars provide cost-effective alternatives, improving patient access and reducing financial burden on healthcare systems. High prevalence, ongoing demand for long-term therapy, and strong payer incentives position autoimmune disorders as the leading application segment in the global biosimilars market.
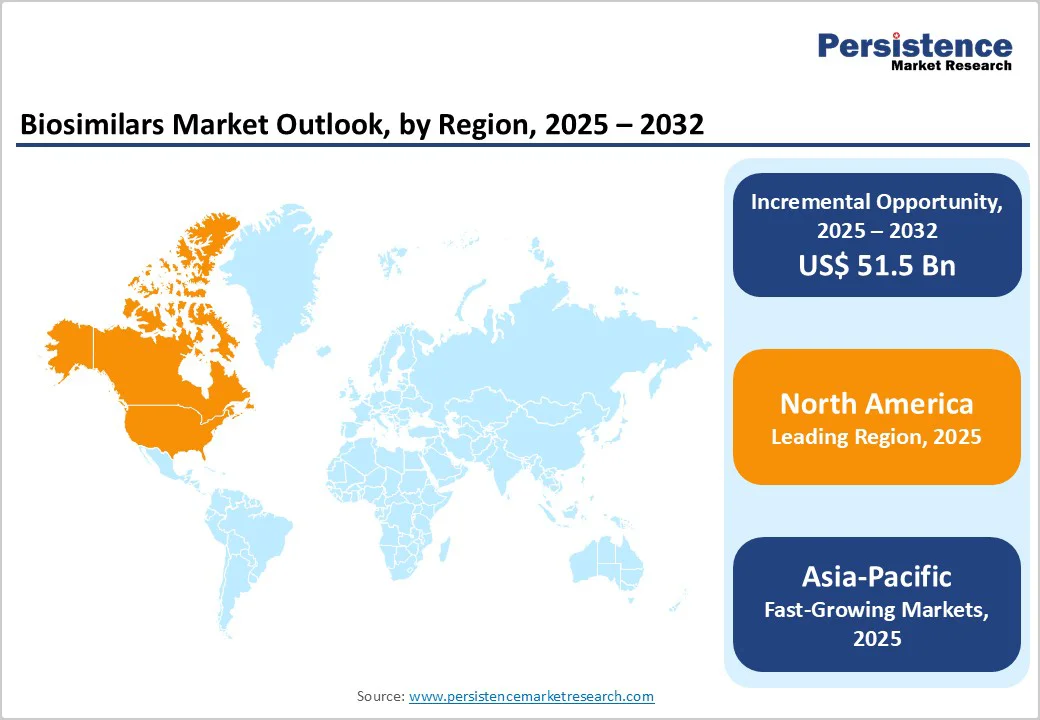
North America dominates the biosimilars market with 41.2% share in 2025, due to strong regulatory support, high-value biologic usage, and payer-driven adoption. The FDA has approved around 76 biosimilars, yet biologics represent only 5% of prescriptions but 51% of drug spending, highlighting their financial impact. Biosimilars have generated over $36 billion in savings for the U.S. healthcare system since 2015, including $12.4 billion in 2023 alone. Regulatory clarity from the Biologics Price Competition and Innovation Act, combined with growing physician and patient awareness, has accelerated uptake. Steep discounts, such as over 80% off some ustekinumab biosimilars, further encourage adoption. High biologic consumption, supportive policies, and cost-saving potential collectively make North America the leading region for biosimilars globally.
Europe's biosimilars market is due to its mature regulatory environment and strong adoption policies. The EMA has approved 86 biosimilar medicines since 2006, ensuring safety and interchangeability with reference biologics. European healthcare systems have benefited from substantial cost savings, exceeding €30 billion by 2022, driven by competition from biosimilars. Policies such as tendering, price-linkage, and prescribing guidelines encourage widespread uptake, particularly in oncology, rheumatology, and diabetes. Countries like Germany, the UK, and France lead in biosimilar penetration, with some products reaching over 90% market share of their reference biologics. Early adoption, regulatory clarity, and cost-containment incentives make Europe an influential and strategic region in the global biosimilars market, both for manufacturers and healthcare payers.
Asia-Pacific is the fastest-growing region in the biosimilars market, driven by supportive regulatory frameworks, expanding manufacturing capacity, and rising demand for affordable biologics. China standardized biosimilar development in 2015, significantly reducing regulatory barriers and accelerating approvals. Japan has also streamlined its biosimilar pathway, approving over 32 biosimilars between 2009 and 2022. The region is rapidly increasing its share of global biomanufacturing, with major facilities in India, China, and South Korea, enabling large-scale production at lower costs. Growing populations, rising incidence of chronic and autoimmune diseases, and increasing healthcare access further drive adoption. Strategic investments in biosimilar R&D, coupled with government initiatives to enhance affordability and accessibility, position the Asia-Pacific region as the fastest-growing and highly attractive market for biosimilars globally.
Leading companies in the biosimilars market focus on high-precision manufacturing, advanced formulation, and robust quality control. They invest in monoclonal antibody and protein biosimilars, optimize production for consistency, and form partnerships with healthcare providers and distributors. R&D emphasizes efficacy, safety, and cost-effectiveness, supporting rising chronic disease treatment, broader patient access, and growing global biosimilar adoption.
The global biosimilars market is projected to be valued at US$ 34.7 billion in 2025.
Rising biologic patent expiries, chronic disease prevalence, healthcare cost pressures, regulatory support, and physician acceptance drive biosimilars.
The global biosimilars market is poised to witness a CAGR of 13.9% between 2025 and 2032.
Opportunities include oncology and immunology biosimilars, interchangeable products, emerging markets expansion, strategic partnerships, and cost-efficient large-scale manufacturing innovations.
Pfizer Inc., Sandoz International GMBH, Eli Lily & Company, Hospira Inc., Amgen, Inc., Biocon Ltd.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Drug Class
By Application
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author