ID: PMRREP4220| 200 Pages | 1 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

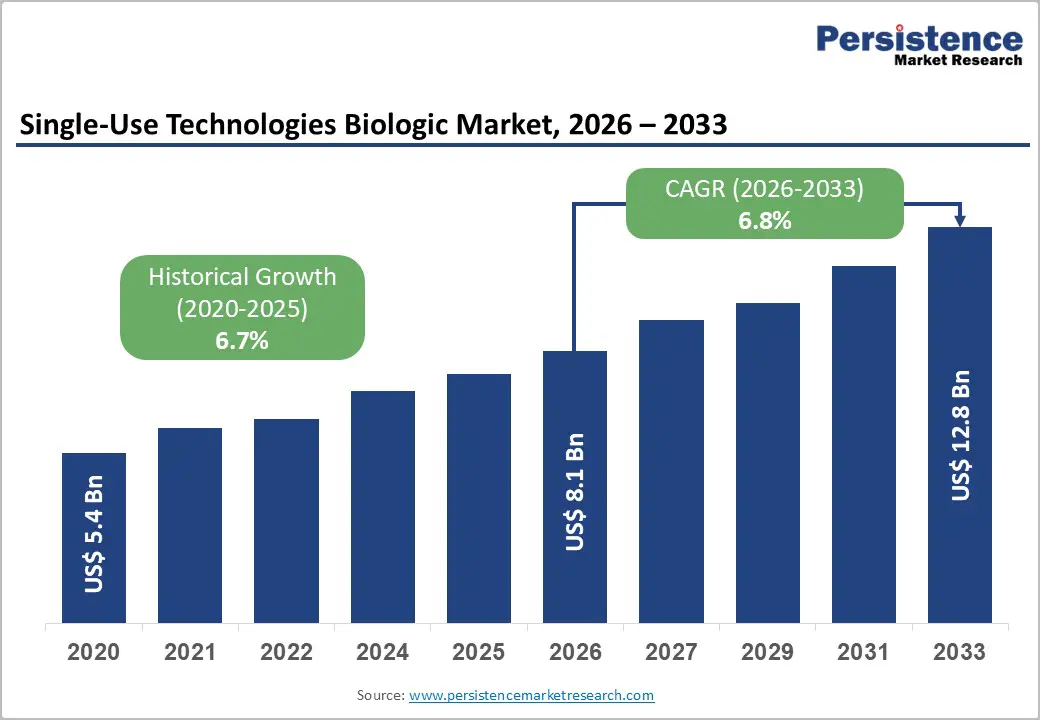
The global single-use technologies biologic market size is likely to be valued at US$ 8.1 billion in 2026, and is projected to reach US$ 12.8 billion by 2033, growing at a CAGR of 6.8% during the forecast period 2026−2033. Market expansion is primarily driven by rising adoption of biologic therapies and advanced bioprocessing techniques. Aging populations and increasing prevalence of chronic and rare diseases create sustained demand for biologics. Integration of automation, digital monitoring, and advanced single-use systems enhances production efficiency and reduces contamination risks, enabling cost-effective operations. Increased clinical awareness and broader adoption of innovative treatment modalities improve accessibility across diverse patient segments.
Development of healthcare infrastructure, including contract manufacturing organizations and hospital-based facilities, supports scalable production capabilities. Regulatory frameworks that facilitate accelerated approvals for single-use systems encourage investment and adoption. Technological advancements, such as modular bioprocessing platforms and disposable components, provide flexible production solutions that accommodate both pilot- and commercial-scale manufacturing requirements.
| Key Insights | Details |
|---|---|
|
Single-Use Technologies Biologic Market Size (2026E) |
US$ 8.1 Bn |
|
Market Value Forecast (2033F) |
US$ 12.8 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
6.8% |
|
Historical Market Growth (CAGR 2020 to 2025) |
6.7% |
Rising Biologic Therapy Adoption
Biologic therapies require complex production processes with strict sterility and quality controls, creating operational challenges for traditional stainless-steel facilities. Conventional systems demand extensive cleaning, sterilization, and validation between production cycles, increasing downtime and operational costs. Single-use technologies employ pre-sterilized, disposable components that eliminate the need for repeated cleaning and reduce the risk of cross-contamination. These systems enable manufacturers to quickly switch between products and adjust production scales to meet changing demand without major infrastructure modifications. The modularity of single-use platforms supports flexible batch sizes and enables efficient resource utilization, reducing both capital and operating expenditures while maintaining high-quality standards.
The fast pace of biologic development, from research through clinical trials to commercialization, places pressure on manufacturers to adopt responsive and efficient production platforms. Single-use technologies facilitate smaller, customized production runs, supporting personalized medicine and early-stage clinical batches without the burden of large-scale stainless-steel facilities. Reduced cleaning and validation requirements enhance productivity and minimize operational downtime, enabling faster delivery of therapies to market. These systems also simplify compliance with regulatory standards for sterility and contamination control, supporting consistent product quality. Operational flexibility and cost efficiency strengthen manufacturers' competitive positioning and enable rapid adaptation to evolving market demands.
Concerns over Extractables and Leachables
Single-use technologies for biologics face critical challenges linked to extractables and leachables, which are chemical substances that can migrate from plastic components into biologic products during manufacturing or storage. These compounds have the potential to compromise product purity, stability, and safety, creating significant regulatory scrutiny. The variability in polymer formulations and the diversity of additives used in single-use systems introduce uncertainties in the types and levels of extractables and leachables. High-sensitivity biologics, such as monoclonal antibodies and cell therapies, are particularly susceptible to chemical interactions, as even trace contaminants can induce aggregation, structural changes, or immunogenic responses.
Process design and operational practices are heavily influenced by the need to control extractables and leachables. Material selection, supplier qualification, and sterilization methods must be carefully aligned to minimize chemical migration. Frequent monitoring and testing during storage, handling, and manufacturing extend timelines and increase operational costs. Differences in regulatory expectations across regions further complicate adoption, as companies must demonstrate comprehensive safety profiles for each component. Supply chain disruptions or variations in single-use component batches can also introduce inconsistencies in chemical leachables, impacting the reproducibility of biologic products.
Integration with Advanced Digital Technologies
The adoption of advanced digital technologies enhances operational efficiency and process reliability in biologic production. Real-time monitoring systems, combined with predictive analytics, allow continuous tracking of critical parameters such as temperature, pH, and flow rates, enabling precise control over production processes. This reduces the risk of batch failures and ensures consistent product quality. Digital twins and simulation tools provide virtual representations of bioprocesses, facilitating scenario testing and optimization without physical disruption. Such integration streamlines process validation, shortens development timelines, and minimizes resource utilization, contributing to cost efficiency and regulatory compliance.
Data-driven automation platforms facilitate seamless connectivity across upstream and downstream operations, improving decision-making and responsiveness to process deviations. Cloud-based systems enable centralized data aggregation, supporting scalable production and cross-site collaboration. Intelligent process control and machine learning algorithms enhance predictive maintenance and reduce equipment downtime, thereby safeguarding continuous operations. The ability to harness high-volume, high-quality data supports regulatory documentation and audit readiness, strengthening operational governance.
Product Type Insights
Single-use bioreactors are projected to account for approximately 40% of the single-use technologies biologic market revenue share in 2026, reflecting their essential role in biologic production. These systems provide flexibility for scaling processes from laboratory to commercial manufacturing without significant infrastructure investment. They enable rapid adjustments in production volume to meet fluctuating demand while maintaining high process efficiency. The disposable nature of critical components minimizes cleaning requirements and reduces turnaround time between batches. Integration with automated sensors and control systems supports real-time monitoring, process optimization, and regulatory compliance. Widespread adoption in contract manufacturing organizations and research institutions underscores their strategic importance in accelerating biologic development pipelines.
Single-use filtration systems are expected to be the fastest-growing segment during the 2026-2033 forecast period, driven by increasing demand for high-purity biologics and continuous processing. These systems provide scalable solutions for virus removal, clarification, and sterile filtration while minimizing the risk of cross-contamination. The modular design allows seamless integration with upstream and downstream single-use equipment, supporting flexible and efficient production workflows. Innovations in membrane technology enhance recovery rates, throughput, and process reproducibility. Real-time monitoring and process analytical technology adoption further improve operational efficiency and ensure product quality, thereby positioning these systems as critical components of modern biologic manufacturing operations.
Material Insights
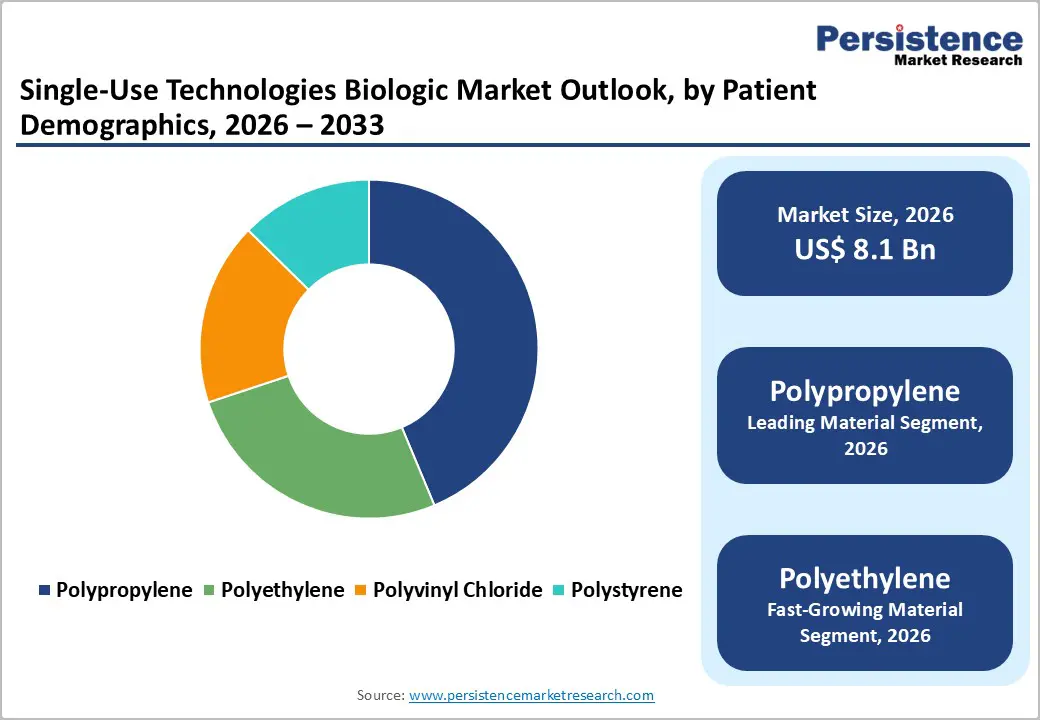
Polypropylene extracts are poised to dominate, with a projected market share of over 45% in 2026, driven by their biocompatibility, chemical resistance, and widespread adoption in single-use components. Polypropylene is widely used in bioreactors, tubing, and filtration assemblies because of its durability under sterilization processes such as gamma irradiation and autoclaving. Its reliability supports consistent product quality and reduces the risk of contamination. High clinical and manufacturing trust in polypropylene ensures preference among contract manufacturers and pharmaceutical companies. Polypropylene integrates effectively with automation systems and digital monitoring, streamlining process control.
Polyethylene is estimated to be the fastest-growing segment from 2026 to 2033, fueled by increased adoption in flexible containers, tubing, and specialized disposable assemblies. Advancements in polymer engineering have enhanced the chemical stability and sterilization tolerance of polyethylene, making it suitable for high-purity applications. Adoption is driven by the increasing use of modular and small-batch bioprocessing systems, where flexibility and cost-effectiveness are essential. Polyethylene's compatibility with digital monitoring sensors and automated systems enhances process tracking and efficiency. Growth in emerging markets and contract manufacturing applications further drives demand.
End-User Insights
Pharmaceutical companies are likely to be the leading segment, with a projected 50% share of the single-use technologies biologic market in 2026, owing to their extensive production infrastructure and demand for high-volume biologic therapies. These companies utilize single-use technologies to enhance process efficiency and ensure consistent product quality across large-scale production. Disposable components reduce the need for complex cleaning and sterilization, minimizing operational downtime. Integration with automated monitoring and digital systems supports regulatory compliance and real-time process control. The scalability of single-use solutions enables rapid responses to market demand and clinical requirements, supporting both commercial biologics and vaccine manufacturing while maintaining operational agility.
Contract manufacturing organizations (CMOs) are anticipated to be the fastest-growing segment from 2026 to 2033, fueled by increased outsourcing of biologic production and demand for flexible manufacturing solutions. CMOs leverage single-use technologies to serve multiple clients efficiently while reducing capital investment in permanent infrastructure. Modular production lines allow small to mid-scale batch manufacturing with minimal setup time. Integration with digital monitoring and process analytics provides real-time data on production performance, quality, and compliance. Strategic collaborations with global pharmaceutical and biotechnology companies further expand service offerings, accelerate project timelines, and enhance operational flexibility, positioning CMOs as critical partners in biologic development and commercial supply.
North America Single-Use Technologies Biologic Market Trends
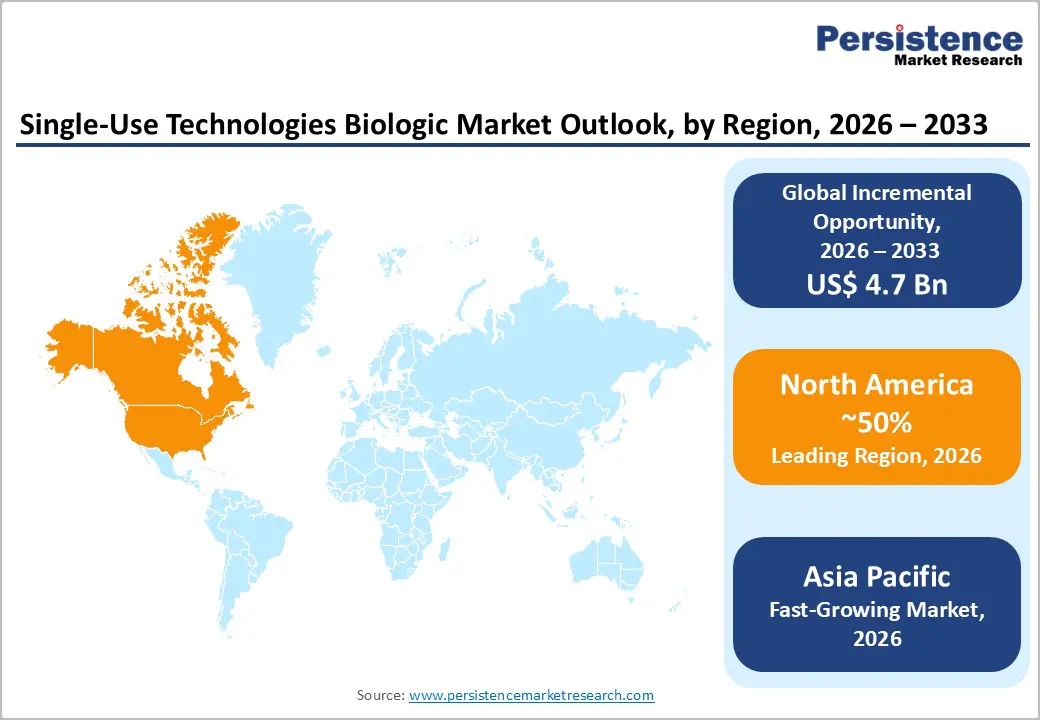
North America is projected to account for approximately 50% of the single-use technologies biologic market share in 2026, reflecting a substantial concentration of high-volume biologic production facilities and well-established pharmaceutical infrastructure. Extensive investment in biologic research and development enables rapid adoption of innovative production technologies, including disposable bioreactors and filtration systems. The presence of leading pharmaceutical and biotechnology companies facilitates early integration of advanced digital monitoring, process analytical technologies, and automation, thereby enhancing process control, minimizing contamination risks, and accelerating scale-up from laboratory to commercial manufacturing. Strong regulatory alignment and robust quality standards further encourage deployment of single-use systems, ensuring compliance while supporting efficient validation processes and consistent product output.
Advanced operational capabilities also contribute to market dominance, with manufacturers leveraging single-use technologies to optimize production cycles and reduce capital expenditure on permanent stainless-steel facilities. High demand for complex biologics, including monoclonal antibodies and vaccines, necessitates flexible, modular manufacturing solutions that can respond to fluctuating production requirements. Contract manufacturing organizations in the area adopt these systems to provide scalable services, creating an ecosystem that supports both innovation and rapid market entry. Integration of real-time data analytics enables predictive maintenance, risk mitigation, and improved resource utilization, reinforcing operational efficiency.
Europe Single-Use Technologies Biologic Market Trends
Europe maintains a strong presence in single-use biologic technologies, supported by well-established pharmaceutical and biotechnology hubs with advanced manufacturing infrastructure. Leading companies in this area have integrated disposable bioreactors, filtration systems, and automated monitoring platforms to enhance operational efficiency, reduce contamination risks, and accelerate product development timelines. High regulatory standards for biologic production, combined with stringent quality control protocols, drive adoption of single-use solutions that facilitate compliance and simplify process validation. Investment in digital monitoring, process analytical technology, and real-time data analytics enables precise control over complex biologic production, supporting consistent output and reliable scale-up from laboratory to commercial manufacturing.
Manufacturers prioritize flexibility and rapid deployment, particularly for mid-scale and specialty biologics, where modular production lines enable adaptation to evolving demand and diversification of the pipeline. Collaboration between contract manufacturing organizations and established pharmaceutical companies fosters knowledge transfer, operational optimization, and access to cutting-edge single-use technologies. Focused government initiatives and research funding promote innovation in disposable systems and process intensification strategies. Advancements in membrane materials, bioreactor design, and integration with intelligent control systems improve product recovery, throughput, and process reproducibility.
Asia Pacific Single-Use Technologies Biologic Market Trends
Asia Pacific is forecasted to be the fastest-growing regional market for single-use technologies in biopharmaceutical manufacturing between 2026 and 2033, driven by the rapid expansion of biopharmaceutical manufacturing capacity and increasing adoption of flexible production solutions. Emerging contract manufacturing organizations are establishing modular facilities that leverage disposable bioreactors, filtration systems, and automated monitoring platforms to serve multiple clients efficiently. These systems enable rapid scale-up from laboratory to commercial production without significant investment in permanent infrastructure, thereby allowing manufacturers to respond quickly to fluctuating demand for vaccines and novel biologics. Government support through funding initiatives and industrial parks for biotechnology accelerates capacity growth and encourages private-sector investment, thereby creating a favorable environment for technology adoption. The growing availability of a skilled workforce and targeted training programs further strengthens operational readiness for single-use manufacturing, thereby enhancing process efficiency and reducing production timelines.
High demand for advanced biologics and the outsourcing of production by global pharmaceutical and biotechnology companies contribute to the accelerated deployment of single-use systems. Integration of digital monitoring, real-time data analytics, and process analytical technology enhances predictive maintenance, quality assurance, and regulatory alignment, supporting consistent output at scale. Manufacturers increasingly prioritize contamination control, product yield optimization, and throughput improvement, and single-use solutions effectively address these requirements. Cost advantages associated with reduced cleaning and sterilization, coupled with faster facility commissioning, make disposable technologies highly attractive for small and mid-scale production.
The global single-use technologies biologic market demonstrates a moderately consolidated structure, with leading companies capturing a significant portion of the overall market. Key players such as Thermo Fisher Scientific, Applikon Biotechnology, GE Healthcare, Pall Corporation, NewAge Industries, and Saint-Gobain S.A. collectively account for approximately 55% of market share, underscoring the dominance of established manufacturers with global operations. These companies leverage extensive product portfolios encompassing disposable bioreactors, filtration systems, and modular process components to meet the diverse requirements of biologic production. Strategic partnerships, mergers, and acquisitions further strengthen competitive positioning, enabling the integration of advanced technologies, digital monitoring solutions, and process analytical tools that enhance operational efficiency and compliance with regulatory standards.
Alongside major players, a variety of smaller and niche suppliers coexist, focusing on specialized applications and tailored solutions to address unique production challenges. These emerging companies contribute to market diversity by offering innovative materials, disposable assemblies, and flexible manufacturing systems that complement the capabilities of larger manufacturers. The market structure encourages continuous technological advancement, with competitive differentiation increasingly driven by the ability to deliver scalable, high-quality single-use solutions that reduce contamination risk, accelerate process validation, and optimize production workflows.
Key Industry Developments
The global single-use technologies biologic market is projected to reach US$ 8.1 billion in 2026.
Rising demand for flexible, scalable, and contamination-controlled biologic manufacturing is driving the market.
The single-use technologies biologic market is poised to witness a CAGR of 6.8% from 2026 to 2033.
Integration of advanced digital technologies and modular production systems is opening lucrative market opportunities.
Some of the key market players include Thermo Fisher Scientific Inc, Applikon Biotechnology B.V., GE Healthcare, Pall Corporation, NewAge Industries, Inc., Saint-Gobain S.A.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 – 2025 |
|
Forecast Period |
2026 – 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Material
By End-User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author