ID: PMRREP13870| 198 Pages | 3 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

The global reprocessed single-use devices market size is likely to value at US$1.9 Bn in 2025 and reach US$4.7 Bn by 2032, growing at a CAGR of 13.8% during the forecast period from 2025 to 2032.
The reprocessed single-use devices market is witnessing robust growth, driven by escalating healthcare costs, sustainability initiatives, and regulatory support for eco-friendly medical practices.
| Key Insights | Details |
|---|---|
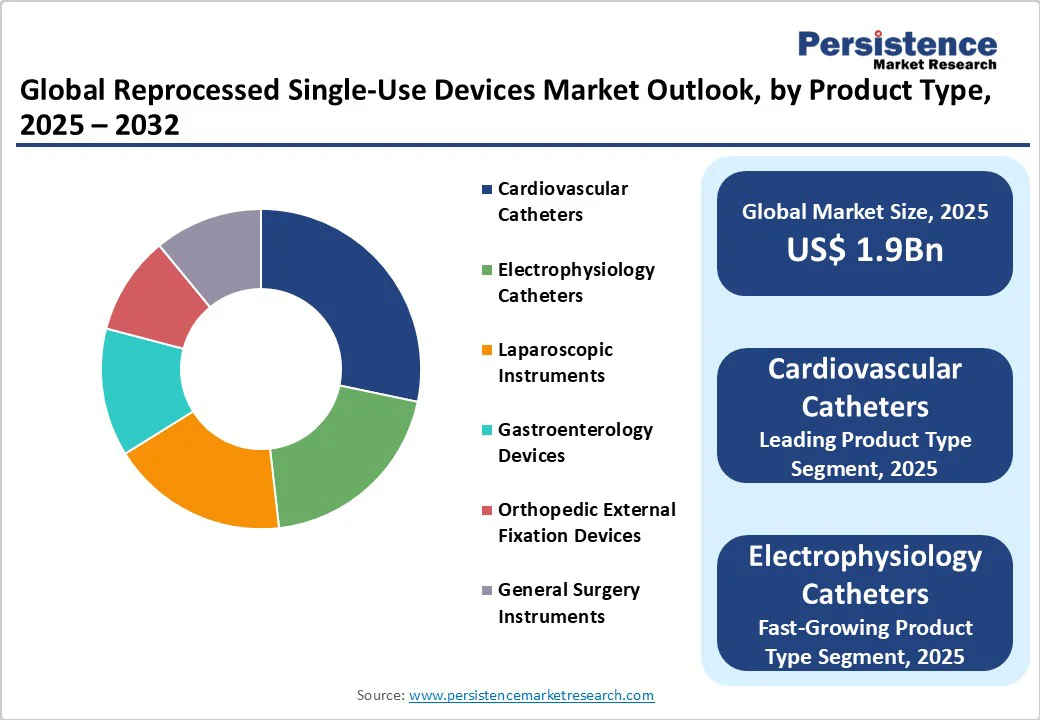
| Reprocessed Single-Use Devices Market Size (2025E) | US$ 1.9Bn |
| Market Value Forecast (2032F) | US$ 4.7Bn |
| Projected Growth (CAGR 2025 to 2032) | 13.8% |
| Historical Market Growth (CAGR 2019 to 2024) | 11.9% |
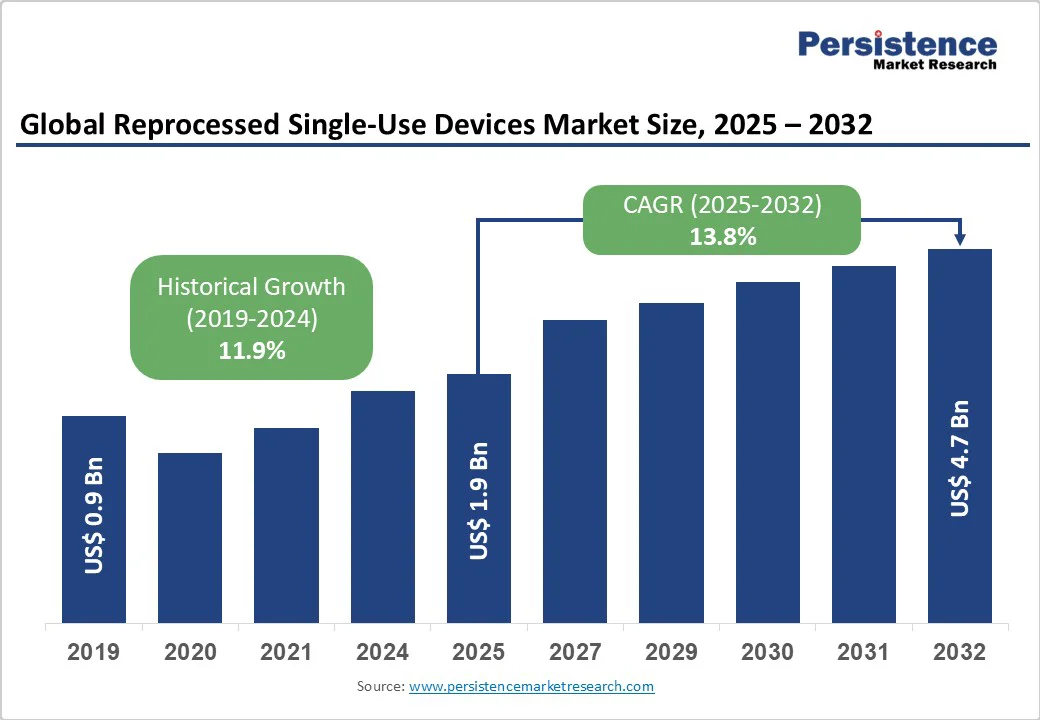
The global reprocessed single-use devices market is experiencing substantial growth due to skyrocketing healthcare expenditures and the imperative for cost optimization in medical facilities worldwide. Reprocessed devices provide a viable alternative to virgin single-use items, reducing expenses by 40-60% without compromising patient safety, as validated by FDA guidelines.
The World Health Organization (WHO) reported that global health spending reached US$9.8 trillion in 2021, accounting for 10.3% of global GDP, with total spending at hospitals accounted for 65% to 84% of total health spending.
According to the Centers for Medicare & Medicaid Services (CMS), U.S. health care spending reached $4.9 trillion in 2023, equating to $14,570 per person., reprocessing programs in facilities such as Mayo Clinic have saved million since 2020.
As healthcare systems worldwide grapple with inflation and resource scarcity, reprocessed single-use devices position themselves as a cornerstone for financial resilience, driving market penetration through 2032 and enabling providers to allocate savings toward advanced treatments and patient care enhancements.
The reprocessed single-use devices market faces significant challenges driven by stringent regulatory requirements and ongoing concerns about safety and efficacy. Regulatory agencies such as the FDA and EU Notified Bodies enforce rigorous validation processes, including microbial contamination testing, material durability assessments, and sterilization efficacy verification. These demanding standards elevate operational costs and complexity, creating substantial barriers for smaller reprocessing firms lacking extensive resources.
Conservative healthcare sectors remain hesitant, further slowing adoption rates. Although technological advancements in traceability and quality assurance have improved safety monitoring and risk mitigation, inconsistent regulatory frameworks across regions hinder seamless growth. The absence of globally harmonized guidelines exacerbates uncertainties, complicating approval pathways and fostering mistrust among providers and patients.
Addressing these regulatory and perception challenges through standardized international protocols is crucial to unlocking the reprocessed single-use devices market’s sustainability potential, reducing medical waste, and enabling broader acceptance of reprocessed single-use medical devices worldwide.
The growing emphasis on sustainability mandates and green healthcare initiatives is creating substantial opportunities for the reprocessed single-use devices market. Governments worldwide are increasingly integrating environmental criteria into healthcare policies, linking reimbursements and funding to providers’ adherence to low-carbon and waste-reduction practices. This regulatory momentum encourages hospitals and clinics to adopt reprocessed devices, which significantly reduce medical waste and carbon emissions.
In addition, insurers are beginning to incentivize sustainable procurement, making eco-friendly devices more financially attractive. Manufacturers are responding by innovating with biodegradable materials and enhanced reprocessing technologies that maintain safety while minimizing environmental impact.
This convergence of policy, economic incentives, and technological advances is expected to drive strong market growth through 2032. Ultimately, the trend supports a circular economy within healthcare, enabling stakeholders to reduce ecological footprints while improving cost efficiency, thereby transforming the industry’s approach to sustainability and patient care.
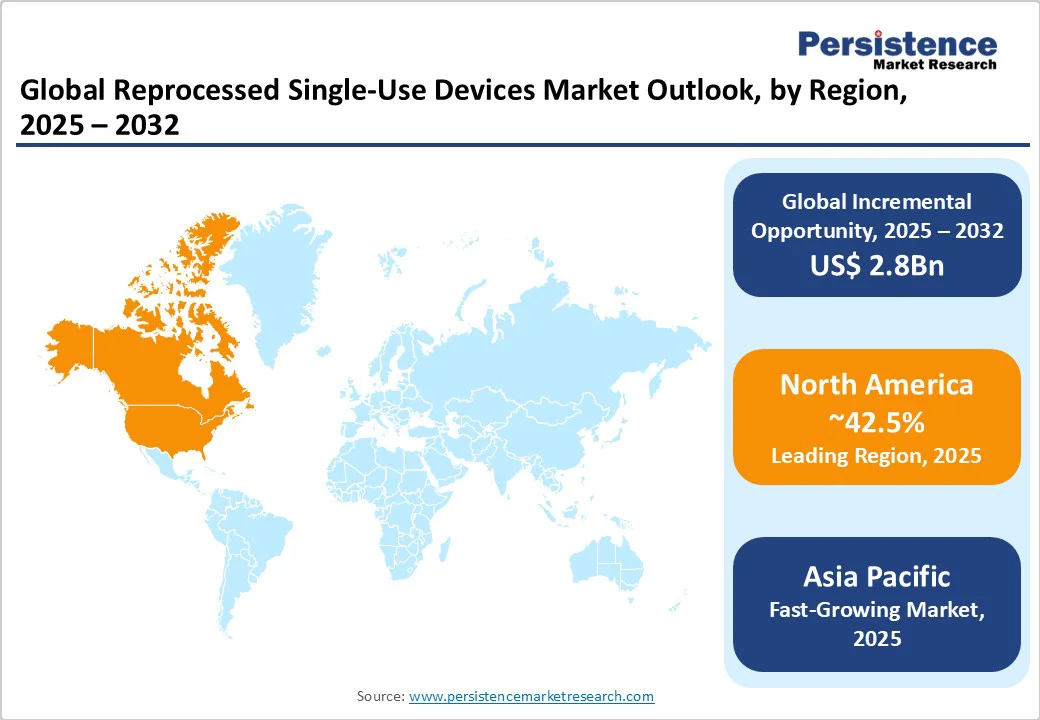
North America is a leading market and is likely to capture a 42.5% share in 2025. This dominance stems from a combination of mature regulatory oversight, high healthcare expenditure, and early adoption trends, particularly in the U.S. and Canada. The U.S. healthcare sector alone reached $4.9 trillion in 2023, according to CMS, underscoring the scale of spending and the urgent need for cost-efficient, sustainable solutions such as device reprocessing.
The Food and Drug Administration (FDA) plays a critical role in ensuring safety, mandating that reprocessed devices meet the same standards as new ones. This regulatory clarity has built trust among providers, encouraging adoption across major hospital systems.
Additionally, supportive policies from insurers and sustainability mandates have further accelerated integration. Canada, following similar environmental and cost-containment priorities, is also expanding its reprocessing infrastructure. Overall, North America's structured approach and innovation-friendly environment continue to drive the region's leadership in this sector.
Asia Pacific is emerging as the fastest-growing region in the reprocessed single-use devices (SUDs) market, driven by rapid healthcare infrastructure development, rising surgical volumes, and increasing environmental awareness. Countries such as China and India are at the forefront of this growth, propelled by large populations and expanding access to surgical care.
In China, the medical equipment market reached 1.27 trillion Yuan (~US$179 billion) in 2023, according to the China Association of Medical Equipment. This growth aligns with national strategies promoting healthcare sustainability, including policies targeting medical waste reduction.
Reprocessing is gaining traction in hospitals, particularly for high-use devices in laparoscopic and minimally invasive procedures. Similarly, India’s focus on reducing healthcare costs while managing waste is fostering a favorable environment for reprocessed SUDs. Both countries are also investing in local reprocessing capabilities and regulatory frameworks. As demand for cost-effective, eco-conscious solutions intensifies, the Asia Pacific is poised to become a central hub for market expansion.
Europe stands as the second fastest-growing region in the reprocessed single-use devices (SUDs) market, propelled by a strong regulatory foundation and a growing emphasis on environmental, social, and governance (ESG) principles. The implementation of the EU Medical Device Regulation (MDR) has established strict standards for safety, performance, and traceability, which reprocessed devices must meet, fostering increased trust among healthcare providers.
In parallel, the European Green Deal and the Circular Economy Action Plan are encouraging hospitals and manufacturers to adopt sustainable practices, including medical device reprocessing, to reduce healthcare waste and lower carbon footprints.
Countries such as Germany, the Netherlands, and the Nordic nations are leading the way, integrating reprocessed SUDs into national procurement strategies and hospital sustainability frameworks. With increasing public and institutional support for environmentally responsible healthcare, the region is seeing rapid adoption across high-volume specialties such as cardiology, gastroenterology, and orthopedics. Europe's policy-driven, sustainability-first approach continues to accelerate market growth and innovation.
The global reprocessed single-use devices market is moderately consolidated, with key players leveraging regulatory expertise, technological innovations, and strategic partnerships to capture share. Dominated by established firms with FDA approvals and global footprints, the landscape features a mix of full-service reprocessors and specialized providers.
Competition centers on quality assurance, cost competitiveness, and sustainability certifications, with leaders expanding via acquisitions and R&D in automated systems. Regional dynamics influence strategies, from U.S.-centric compliance to Asia’s volume scaling.
The Reprocessed Single-Use Devices market is projected to reach US$1.9 Bn in 2025.
Escalating healthcare costs and sustainability mandates in medical waste reduction are the key market drivers.
The Reprocessed Single-Use Devices market is poised to witness a CAGR of 13.8% from 2025 to 2032.
Green healthcare initiatives and rising outpatient procedures present key market opportunities.
Stryker, Cardinal Health, Medline ReNewal, and Innovative Health are key market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn/Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Class
By End-use
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author