ID: PMRREP35234| 190 Pages | 23 Apr 2025 | Format: PDF, Excel, PPT* | Healthcare

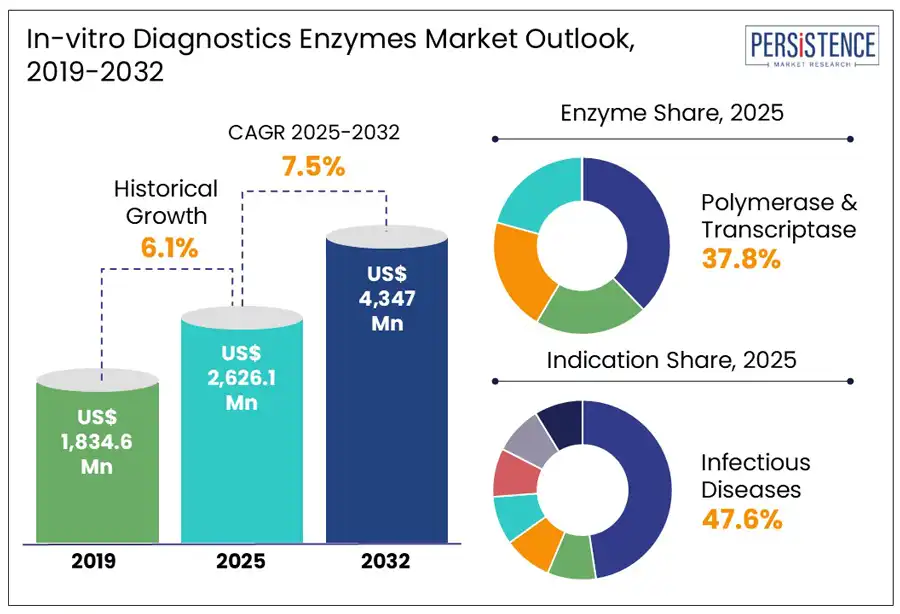
According to the Persistence Market Research report, the global in-vitro diagnostics enzymes market is estimated to grow from US$ 2.6 Bn in 2025 to US$ 4.3 Bn by 2032. The market is projected to record a CAGR of 7.5% during the forecast period from 2025 to 2032.
In-vitro diagnostics (IVD) enzymes play a crucial role in the global healthcare landscape, driving accurate and efficient medical testing. These enzymes are widely used across various disciplines such as molecular diagnostics, immunoassays, and clinical chemistry. They play a vital role in laboratory-based testing of biological samples, enabling detection, monitoring, and management of various medical conditions.
As a biological catalyst, IVD enzymes facilitate biochemical reactions serving as essential reagents in these diagnostic procedures. Commonly used enzymes include reverse transcriptase, and polymerases, which are integral to various testing methods. Growing automation of diagnostic platforms, expanding research and development (R&D) investments in the biotechnology sector, and the development of high-fidelity enzymes are expected to fuel the market growth significantly.
Key Highlights
|
Global Market Attribute |
Key Insights |
|
In-vitro Diagnostics Enzymes Market Size (2025E) |
US$ 2.6 Bn |
|
Market Value Forecast (2032F) |
US$ 4.3 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.5% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.1% |
The growing application of molecular diagnostic techniques - particularly enzyme-assisted nucleic acid amplification technologies (NAATs) - has significantly expanded the in-vitro diagnostics enzymes market. Enzymes such as DNA polymerases including Taq polymerase are widely utilized in Polymerase Chain Reaction (PCR) techniques for disease detection and in cancer genomics. Increased demand for real-time PCR (RT-PCR), especially during outbreaks such as COVID-19, further highlights the vital role of enzymes in achieving high diagnostic accuracy.
To cater to this growing need for enzyme-driven diagnostics, global companies such as Roche and Qiagen are developing innovative technologies. In June 2024, Roche launched NucleoMix, 400 mM PCR product, while Qiagen introduced QIAcuityDx Digital PCR System for oncology diagnostics in September 2024. These advancements in PCR techniques enhance molecular diagnostic capabilities, further underscoring the increasing reliance on enzymes for rapid and accurate diagnostic results.
The in-vitro diagnostics enzymes market faces several restraints challenging its growth and scalability. Risk of contamination in enzyme-based tests and enzyme instability during storage and transport are a few concerns likely to compromise the diagnostic performance, especially in resource-limited settings.
Enzymes being inherently sensitive to humidity, temperature, and pH fluctuations, require cold-chain logistics to retain their activity. Furthermore, contamination, particularly in high-throughput or multi-step manual workflows, can lead to unreliable results, limiting the adoption of enzyme-based tests.
Moreover, regulatory requirements add further complexity for the developers of enzyme-based diagnostic products. They navigate through evolving global standards and stringent quality validation requirements. Achieving consistent enzyme performance across batches is critical for regulatory approval yet difficult to maintain. This remains a major hurdle further complicating assay reproducibility. All the factors relay considerable pressure on assay manufacturers to remain competitive in a rapidly evolving diagnostics market.
The in-vitro diagnostics enzymes market is experiencing increasing demand for high-performance enzyme formulations in point-of-care (POC), molecular diagnostics and immunoassays applications. This, in turn, creates opportunities for companies manufacturing specialized enzymes, including contract manufacturers (CMOs and CDMOs). Diagnostic companies such as Abbott and Roche are increasingly outsourcing the production of enzyme reagents and molecular diagnostic components to CMOs, leading to a more efficient scale-up process.
Additionally, growing integration of automation in diagnostic platforms, together with advancements in bioengineering is transforming the IVD sector. The development of CRISPR-based diagnostics is further opening new frontiers for next-generation enzyme reagents tailored for precision diagnostics.
Several research groups and startups, including Sherlock Biosciences and Mammoth Biosciences (DETECTR), have developed CRISPR diagnostic platforms. These genome-editing CRISPR/Cas systems use programmable nucleic acid detection by engineering Cas12 and Cas13 enzymes to detect pathogens or mutations. Overall, the IVD enzyme sector presents a growing range of opportunities for manufacturers, driven by collaborations, in-house innovation, and expanding global demand for enzyme-based testing.
The polymerase & transcriptase enzymes segment is projected to hold a revenue share of 37.8% in 2025 within the global in-vitro diagnostics enzymes market. These enzymes play a crucial role in molecular biology, genetics, and medicine. Reverse transcriptases are a valuable tool for genomics and gene expression studies as well as molecular diagnostics and are used within molecular biology workflows, including PCR, cloning and sequencing.
The infectious diseases segment is expected to dominate the indication category in 2025, with 47.6% of the global in-vitro diagnostics enzymes market share. The segment’s dominance is owed to the high burden of infectious pathogens globally and the growing need for rapid diagnosis. Recent studies indicate infectious diseases account for 28% of the global burden of diseases, with bacterial infections contributing half of this total.
Unlike chronic conditions such as cardiovascular diseases and diabetes, infectious disease diagnostics need immediate detection and testing, often requiring enzyme-based techniques such as PCR and isothermal amplification. The growing availability of molecular diagnostics and POCT in emerging countries supports this segment’s leading market share over other indications.
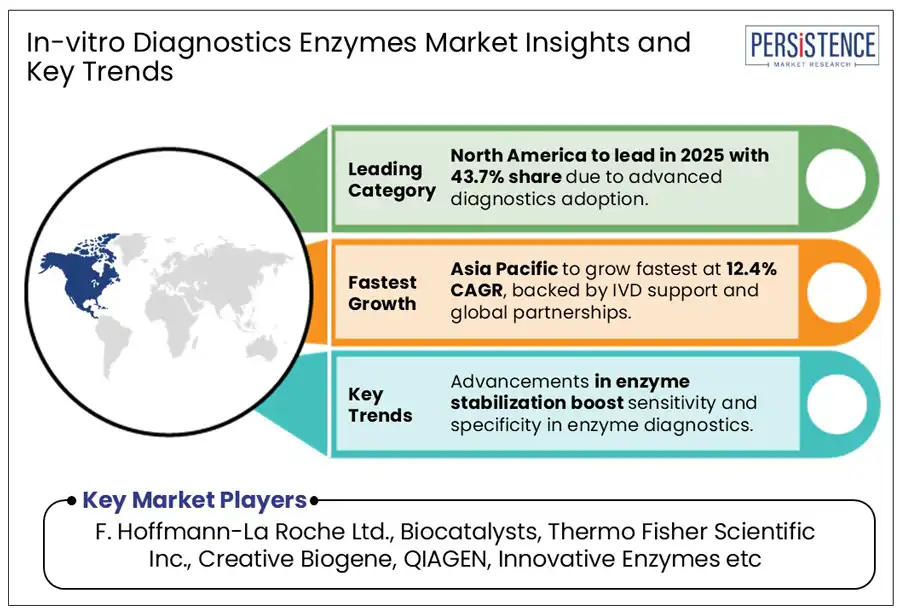
North America is anticipated to hold 43.7% of the global market share in 2025, driven by increasing adoption of enzyme-based technologies for cancer detection.
The U.S. in-vitro diagnostic enzymes market plays a key role in North America’s dominance. Rising cancer incidence in the region drives the demand for innovative diagnostic solutions to enable early detection and personalized treatment. The American Cancer Society estimates 2,041,910 new cancer cases and 618,120 cancer deaths in the U.S. in 2025. Enzyme-assisted techniques such as PCR and digital PCR (dPCR) are critical for the precise identification of genetic mutations and cancer biomarkers. Moreover, increasing collaborations between leading manufacturers further fuel the innovation in the molecular diagnostics sector in the region.
In September 2024, Maravai LifeSciences companies TriLink BioTechnologies and Alphazyme collaborated to launch CleanScribe™ RNA Polymerase enzyme to reduce double-stranded RNA (dsRNA) in mRNA production, enhancing mRNA therapeutics production.
Europe is expected to witness fast-growth globally, and estimated to account for a share of 26.5% in 2025. Europe is a key market for leading biotech firms and research institutions, driving innovation and advancement in genomics and molecular research.
Increasing investments by government and private institutions in the IVD and healthcare sector is driving the IVD enzymes market in Europe. Germitec, in collaboration with the European Investment Bank (EIB), signed a €25 million agreement under InvestEU's research and development policy in November 2023. The project aimed to develop and commercialise new products related to medical devices, pharmaceuticals, diagnostics, and advanced therapy medicinal products.
Similarly, an EU-driven initiative was undertaken on December 2024, streamlining regulatory processes for combined studies (IVDs, medical device, and medicinal products) through the COMBINE Programme. Furthermore, in September 2023, Qiagen expanded its enzyme portfolio, offering enhanced solutions to support life science research labs globally, providing essential tools for genomic and molecular research advancements.
Hence, the emphasis on regulatory standards allows quicker market entry for enzyme-based diagnostic solutions, further accelerating innovation and growth in Europe.
Asia Pacific market for in-vitro diagnostics enzymes is estimated to grow by 12.4% during the forecast period. Growing emphasis on molecular diagnostic results for disease detection has led to an increase in IVD equipment and reagents, including enzymes, across smaller setups. Countries such as Japan and India have witnessed a paradigm shift in the IVD sector in the recent years.
The National Health Mission launched by the Indian government has proposed the establishment of more diagnostic labs and hospitals, driving the diagnostic market growth in Indian towns. In line with this, the Department of Health Research (DHR) implemented a Central Sector Scheme in December 2024 to establish advanced Viral Research & Diagnostic Laboratories (VRDLs), covering a wider range of infectious diseases. Such initiatives are expected to enhance the diagnostic infrastructure.
Similarly, the Japan in-vitro diagnostics enzymes market is experiencing robust growth driven by its superiority quality of IVD equipment and reagents. Japan has long been at the forefront of introducing superior IVD technologies across the globe. Its emphasis on precision medicine, efficient healthcare delivery, and increasing collaboration with international entities positions the country to maintain a strong influence on the industry. A notable example is the collaboration between Fujirebio Holdings (Japan) and Agappe Diagnostics Ltd (India) in January 2024 for contract manufacturing of cartridge-based CLIA system reagents for the Mispa i60 and Mispa i121 immunology analyzers further strengthening Japan's role in the global market.
The global in-vitro diagnostics enzymes market is moderately fragmented with key players offering specialized products. Growing collaboration to develop novel enzymes and contract manufacturing trends contribute to market growth.
The global market is set to reach US$ 2.6 Bn in 2025.
The market is projected to record a CAGR of 7.5% during the forecast period from 2025 to 2032.
Increasing R&D investments, development of specialized enzymes, and growing advancements in diagnostic platforms is expected to drive the global market.
North America is projected to dominate the global market in 2025.
F. Hoffmann-La Roche Ltd., Biocatalysts, Thermo Fisher Scientific Inc., Creative Biogene, QIAGEN, and Innovative Enzymes are a few leading players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Mn/Bn Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Enzyme:
By Indication:
By Technology:
By End-user:
By Region:
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author