ID: PMRREP35504| 193 Pages | 22 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

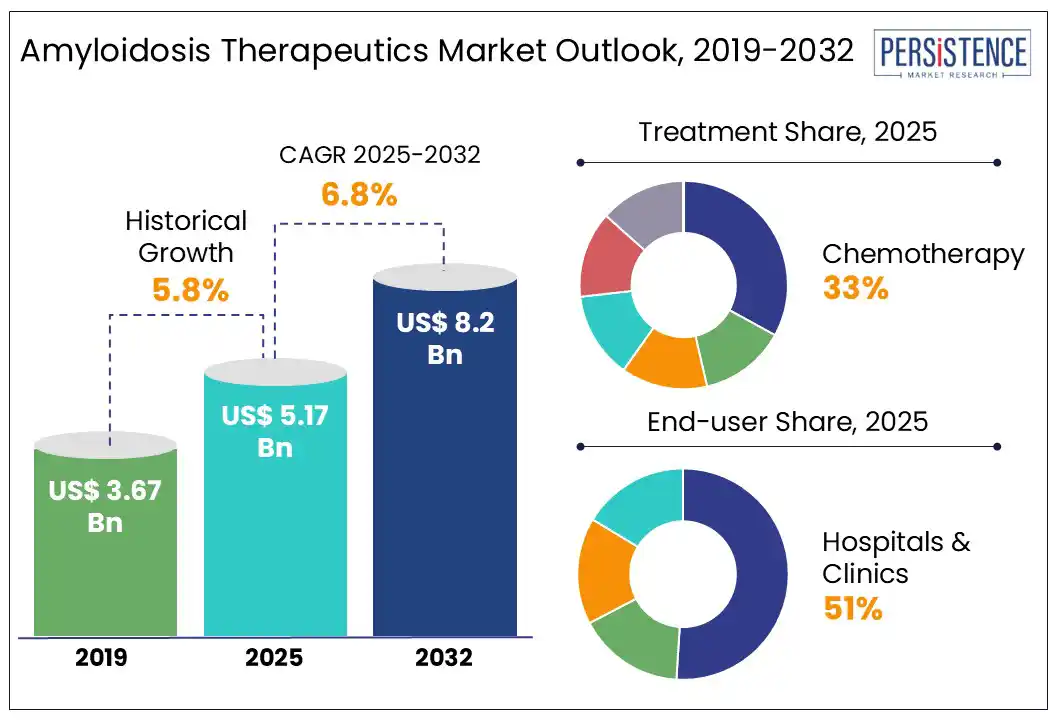
The global amyloidosis therapeutics market size is likely to be valued at US$ 5.17 Bn in 2025 and is estimated to reach US$ 8.20 Bn in 2032, growing at a CAGR of 6.8% during the forecast period 2025 - 2032.
Amyloidosis results from the buildup of amyloid proteins in various organs. There are several types of amyloidosis, such as AL (primary), AA (secondary), hereditary (familial), wild-type (senile), and localized amyloidosis, each affecting different organs such as the heart (cardiac amyloidosis (CA), kidneys (renal amyloidosis), liver, and nerves. Risk factors include age, chronic inflammatory diseases, family history, and long-term dialysis. Left untreated, amyloidosis can lead to serious complications, including heart failure, kidney damage, and nerve dysfunction, making early diagnosis and subtype identification essential for proper treatment. The amyloidosis therapeutics market growth is driven by the rising prevalence and awareness of amyloidosis, early disease detection, and advancements in targeted & personalized therapies.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Amyloidosis Therapeutics Market Size (2025E) |
US$ 5.17 Bn |
|
Market Value Forecast (2032F) |
US$ 8.20 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
6.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.8% |
Early diagnosis of Cardiac Amyloidosis (CA) is critical, as delayed recognition significantly worsens prognosis due to the disease's progressive nature and high mortality. At many centers, the diagnostic process starts with clinical suspicion, often through findings such as unexplained left ventricular hypertrophy or diastolic dysfunction on echocardiography, or non-specific late gadolinium enhancement (LGE) patterns on cardiac MRI.
Amyloidosis often goes unnoticed as its symptoms mimic those of common conditions, making early diagnosis critical to prevent irreversible organ damage. Diagnostic evaluation begins with blood and urine analyses for abnormal proteins, as well as kidney and thyroid function tests. A biopsy taken from abdominal fat, bone marrow, or an affected organ, such as the liver or kidney, also confirms amyloid deposits and determines the type.
Imaging tests and echocardiography assess heart function and its characteristic damage. Cardiac MRI provides detailed images of organ structure and function, and nuclear imaging using radiotracers can identify early cardiac involvement and differentiate between amyloid types, guiding targeted treatment strategies. In transthyretin amyloidosis (ATTR), bilateral carpal tunnel syndrome may occur 5-15 years before cardiac symptoms.
Despite broader reimbursement policies in many regions, the high annual cost of amyloidosis therapies remains a major barrier to patient access and treatment accessibility. Disease-modifying therapies, particularly for ATTR amyloidosis, such as tafamidis, patisiran, vutrisiran, and other emerging agents, can cost over US$ 200,000 to US$ 500,000 annually, depending on the drug and healthcare system. These high prices place a significant strain on national healthcare budgets, insurers, and patients, especially in countries with limited funding frameworks and inconsistent reimbursement mechanisms.
In the U.S., tafamidis (Vyndaqel/Vyndamax) is priced at approximately US$ 225,000 annually per patient, making it financially inaccessible for many despite FDA approvals. A cost-effectiveness analysis found that tafamidis would need a price reduction of over 90% to meet standard U.S. thresholds for value-based care. Similarly, gene-silencing therapies such as patisiran and inotersen are priced even higher, around US$ 450,000 per year, making them out-of-the-way.
The integration of AI, particularly advanced techniques including deep-learning convolutional neural networks (CNNs), has shown promising potential. AI applications across various modalities, such as echocardiography, cardiac MRI, and nuclear imaging, help identify high-risk patients earlier, improving diagnostic accuracy and providing prognostic insights. Scintigraphy is a nuclear medicine imaging technique usually used to diagnose heart conditions. When paired with AI, the system showed diagnostic accuracy similar to that of medical experts. Patients flagged by the AI as having CA showed a twofold increased risk of death and over 17-fold higher risk of heart failure.
CA remains underdiagnosed due to its nonspecific symptoms and the complexity of its diagnosis, but AI offers the potential to improve detection. Early diagnosis is critical for timely treatment.
An international research team led by MedUni Vienna has developed a new AI system that can automatically and reliably detect CA using scintigraphy imaging data. This AI tool was trained and validated on datasets from 16,000 patients across nine institutions in Europe and Asia, including Vienna General Hospital, collected between 2010 and 2020.
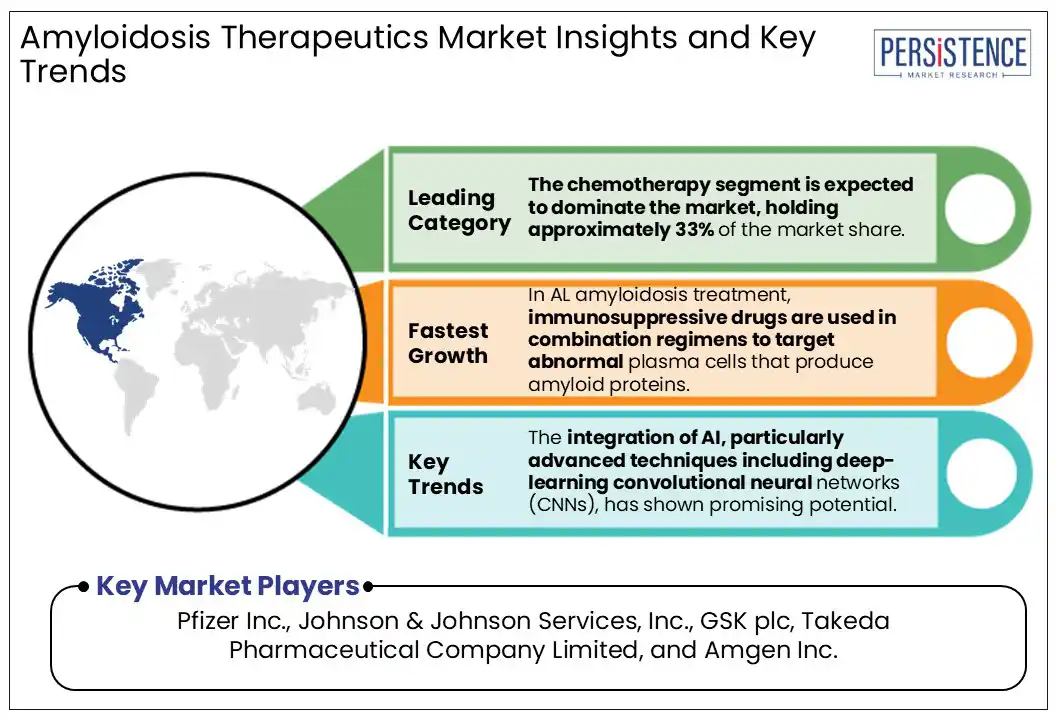
By treatment, the chemotherapy segment is expected to dominate the market, holding approximately 33% of the market share in 2025. In AL amyloidosis, misfolded immunoglobulin light chains form amyloid fibrils that deposit in organs such as the heart, kidneys, and liver, making chemotherapy essential to target the underlying clonal plasma cells and halt amyloid buildup. Treatment involves high-dose melphalan combined with autologous stem cell transplant (ASCT). Novel agents such as proteasome inhibitors (bortezomib) and immunomodulatory drugs have also become frontline therapies, with monoclonal antibodies such as daratumumab, approved by the FDA in 2021.
The immunosuppressive drugs segment is likely to be the fastest-growing over the forecast period. In amyloidosis treatment, immunosuppressive drugs have evolved as part of combination regimens aimed at controlling the abnormal plasma cell clone responsible for amyloid production. These drugs, including corticosteroids (dexamethasone) and newer ones called IMiDs (lenalidomide and pomalidomide), help by reducing the production of harmful proteins (amyloidogenic light chains) and lowering inflammation. They are often combined with chemotherapy agents such as proteasome inhibitors to enhance efficacy.
By end-user, the hospitals & clinics segment is expected to dominate in 2025, accounting for around 51% of the market share. The diagnosis of Amyloidosis involves advanced imaging, biopsies, and laboratory tests, which require sophisticated equipment typically available in hospitals. Treatment involves administering chemotherapy, immunotherapy, or novel targeted drugs, which require expert medical supervision. Hospitals have hematologists, cardiologists, and nephrologists to address the disease complications. The treatment also involves access to chemotherapy, stem cell transplants, and novel drugs including tafamidis and daratumumab. Mayo Clinic, National Amyloidosis Centre, and Vienna General Hospital integrate complex diagnostics and treatments.
The home care segment for amyloidosis treatment and management is growing rapidly, driven by patient preference for bespoke, convenient, and cost-effective care. This trend is more common in North America and Europe, with a high incidence of aging populations and the incidence of chronic diseases such as amyloidosis. Hospitals collaborate with home care providers to ensure continuity of care, especially for patients requiring long-term treatment, such as those with cardiac or systemic amyloidosis. Mayo Clinic in the U.S. employs remote patient monitoring tools and telemedicine consultations. The National Amyloidosis Centre in the UK coordinates with community nursing services.
North America is estimated to dominate the market in 2025, accounting for a market share of approximately 47% in 2025, due to its robust healthcare system and increasing disease awareness. Key growth factors include the approval of novel therapies such as Alnylam's Amvuttra for ATTR-CM, which has expanded treatment options for patients. The integration of AI in diagnostics, along with the FDA clearance of Ultromics’ EchoGo Amyloidosis, is enhancing early detection. The Amyloidosis Program of Calgary (APC) offers multidisciplinary care, patient education, and research engagement, thereby providing a comprehensive care pathway across southern Alberta, Canada.
The U.S. market is experiencing significant growth, driven by advancements in diagnostics, favorable insurance programs, treatment options, and supportive healthcare infrastructure. This growth is supported by increased clinical trials, research investments, favorable insurance programs, and advancements in telehealth & home health monitoring services. BridgeBio’s Acoramidis (Attruby), an oral TTR stabilizer approved in the U.S. in November 2024, is priced at approximately US$ 18,759 per 28-day supply and is positioned as a lower-cost alternative to Pfizer’s tafamidis.
Europe is experiencing significant growth, driven by advancements in treatment options, supportive healthcare infrastructure, and robust regulatory frameworks. Key countries contributing to this expansion include Germany, France, Italy, and the U.K., collectively known as the EU4. Tafamidis (Vyndaqel/Vyndamax), vutrisiran (Amvuttra), and eplontersen (Wainzua) have received regulatory approvals in the EU. The UK's National Amyloidosis Centre, tracking 6,400 patients, illustrates the region's commitment to amyloidosis care. Major therapeutic advancements include the approval of vutrisiran (Amvuttra) by the European Medicines Agency in September 2022 for hereditary transthyretin-mediated amyloidosis, and the approval of acoramidis (Attruby) for transthyretin amyloid cardiomyopathy.
Germany amyloidosis therapeutics market is projected to experience the highest growth within Europe, particularly in the treatment of ATTR. The country's 12 university hospitals monitored over 10,500 amyloidosis patients in 2023, highlighting its commitment to managing rare diseases. Additionally, the Federal Joint Committee (G-BA) ensures that effective treatments receive appropriate industry access, further enhancing the therapeutic landscape.
Asia Pacific amyloidosis therapeutics market is experiencing rapid growth, driven by expanding healthcare infrastructure, regulatory innovation, and rising disease awareness. Key countries including China and Japan are leading this growth. Government initiatives, including Japan’s widespread use of advanced diagnostic tools such as cardiac PYP scans, have significantly improved early detection and treatment of cardiac amyloidosis. Accelerated drug approvals, including for acoramidis and vutrisiran, and increasing adoption of RNA-based therapies are also propelling market expansion. The region is seeing a surge in clinical trials and investments, making it a hub for amyloidosis innovation.
China’s amyloidosis therapeutics market is witnessing a high growth. China’s inclusion of amyloidosis on its National Rare Disease List and NMPA’s faster approval pathways and stronger orphan drug policies have boosted early diagnosis and treatment. NMPA approved Darzalex Faspro (daratumumab) for primary light chain (AL) amyloidosis, making it an officially authorized treatment in China.
The competitive landscape of the amyloidosis therapeutics market is characterized by a mix of established pharmaceutical companies and emerging biotech firms focusing on targeted therapies. Key players such as Pfizer, Johnson & Johnson (Janssen), Takeda Pharmaceutical, Prothena Corporation, and Ionis Pharmaceuticals are leading the development of innovative treatments, particularly for AL and ATTR amyloidosis. Companies are investing in R&D and adopting growth strategies such as product innovations, strategic partnerships, and acquisitions.
The amyloidosis therapeutics market is projected to be valued at US$ 5.17 Bn in 2025.
Market growth is driven by the rising prevalence and awareness of amyloidosis, early disease detection, and advancements in targeted & personalized therapies.
The amyloidosis therapeutics market is poised to witness a CAGR of 6.8% from 2025 to 2032.
The integration of AI, particularly advanced techniques including deep-learning convolutional neural networks (CNNs), has shown promising potential.
Major players include Pfizer Inc., Johnson & Johnson Services, Inc., GSK plc, Takeda Pharmaceutical Company Limited, and Amgen Inc.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author