ID: PMRREP35329| 190 Pages | 19 May 2025 | Format: PDF, Excel, PPT* | Healthcare

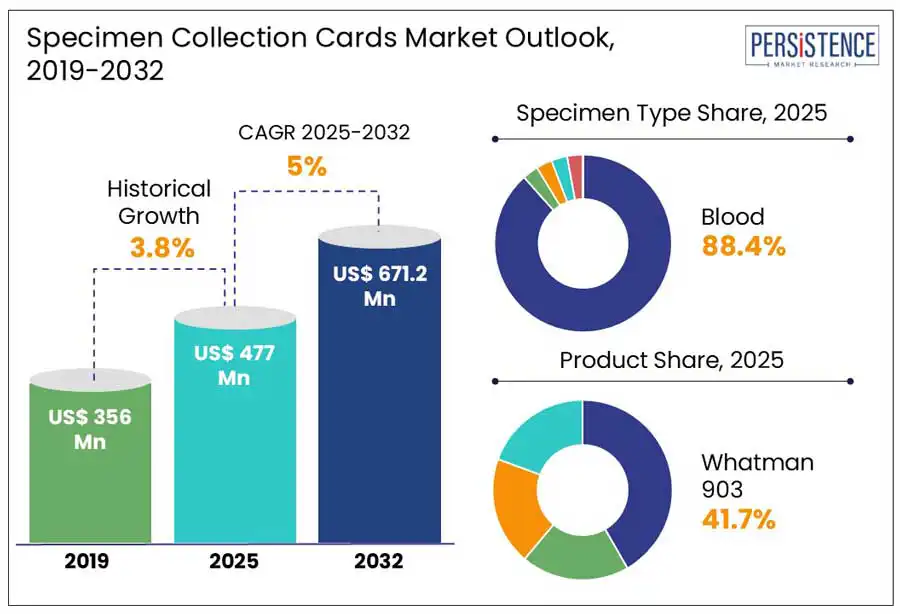
The global specimen collection cards market size is predicted to reach US$ 671.2 Mn in 2032 from US$ 477.0 Mn in 2025. It will likely witness a CAGR of around 5.0% in the forecast period between 2025 and 2032.
Specimen collection cards are extensively used in genomics, diagnostics, pharmacokinetics, and forensic science. Their ability to simplify sample handling by enabling long-term storage at ambient temperatures is expected to drive demand in clinical settings. Their role has extended beyond newborn screening, backed by their ability to stabilize analytes, including small molecules, DNA, and RNA. As healthcare professionals move toward remote sampling and decentralized diagnostics, these cards are poised to become significant to field-based studies, bio-banking initiatives, and large-scale screening programs.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Specimen Collection Cards Market Size (2025E) |
US$ 477.0 Mn |
|
Market Value Forecast (2032F) |
US$ 671.2 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
5.0% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.8% |
The booming DNA and RNA sample preparation market is projected to propel demand for specimen collection cards through 2032, finds Persistence Market Research in a new report. Cards treated with nucleic acid-stabilizing chemicals enable the ambient storage of DNA and RNA without immediate refrigeration or processing. This capability is considered highly valuable in field-based research studies and decentralized healthcare models where cold chain logistics are expensive. A 2023 study published in PLOS ONE validated that Fast Technology for Analysis (FTA) cards are capable of preserving RNA integrity for more than two weeks at ambient temperatures. This has made them ideal for collecting samples in rural or resource-limited areas for later molecular analysis.
The surge in at-home genetic testing is further envisioned to boost this trend. Companies such as Myriad Genetics and 23andMe have been utilizing specimen collection cards to enable customers to self-collect saliva or buccal samples and mail them back to labs without spoilage. Public health initiatives, including newborn screening for inherited disorders, are also embracing Dried Blood Spot (DBS) cards to extract DNA for PCR-based assays, allowing for affordable early intervention programs.
The hematocrit bias, i.e., differences in cell-to-plasma ratio and blood viscosity, is predicted to be a key hindrance to the specimen collection cards market growth. This bias specifically affects the uniformity of blood spreading on filter paper, resulting in inconsistencies in analyte concentration. It further leads to lower analytical accuracy, mainly for quantitative assays in hormone testing, therapeutic drug monitoring, and pharmacokinetics. This constraint will likely deter the wide clinical use of DBS cards in regulated diagnostic settings that require reproducibility and precision.
A 2023 study published in Clinical Chemistry and Laboratory Medicine mentioned that hematocrit levels ranging from 20% to 65% are projected to cause up to 35% variation in analyte quantification from DBS punches. Such variability is poised to undermine confidence in the technology for applications such as antiretroviral drug monitoring in HIV patients or pediatric hormone screening. This is because in such cases, even minor changes can alter treatment decisions. Consequently, this limitation is envisioned to support the specimen processing equipment market as laboratories incline toward automated solutions that reduce variability.
Increasing demand for forensic toxicology testing, especially in workplace drug testing, postmortem investigations, and drug abuse monitoring, will likely create new opportunities for specimen collection card manufacturers. Dried matrix spot cards are expected to gain momentum as these provide a tamper-evident, easy-to-store, and non-invasive method of collecting and preserving biological samples, including saliva, urine, and blood for toxicological analysis. As designer drugs, psychoactive substances, and synthetic opioids proliferate, forensic laboratories are anticipated to shift toward novel collection methods that enable stable sample retention at room temperature. This is mainly valuable for postmortem samples or crime scene sample collection in remote areas.
The UN Office on Drugs and Crime (UNODC) stated in its 2023 report that synthetic opioids contributed to more than 70% of drug-related deaths worldwide. This spurred demand for accessible, widespread toxicology screening solutions. Several law enforcement bodies and public health agencies in Europe and the U.S. hence started integrating Dried Urine Spot (DUS) or DBS cards into field testing kits. The National Institute on Drug Abuse (NIDA), for instance, has been funding research into portable toxicology workflows using DBS cards. It aims to mail these cards to centralized labs, thereby improving access and reducing turnaround time in under-resourced regions.
In terms of specimen type, the market is segregated into blood, saliva, urine, and buccal cells. Among these, blood samples are expected to lead with a share of around 88.4% in 2025. This is attributed to the ability of DBS cards to provide a cost-effective and minimally invasive alternative to venipuncture, which is beneficial in remote, geriatric, and pediatric populations. These can be easily stored and transported at room temperature without the requirement of a cold chain. Newborn screening programs are exhibiting a high demand for blood-based diagnostics. According to the International Society for Neonatal Screening, as of 2024, more than 80 countries utilize DBS cards for routine newborn metabolic screening. This method allows for the early detection of conditions, including sickle cell disease, congenital hypothyroidism, and phenylketonuria.
Saliva samples, on the other hand, are poised to witness a steady CAGR from 2025 to 2032. This is due to their rising validation in molecular diagnostics, ease of self-collection, and non-invasive nature. Saliva is considered a significant alternative to blood for hormone, RNA, and DNA analysis. This has made it ideal for population-scale screening programs and at-home testing kits. Companies such as Everlywell, CircleDNA, and AncestryDNA have been shifting toward saliva-based collection cards to enhance user compliance and logistics. The rapid expansion of direct-to-consumer (DTC) genetic and lifestyle testing as well as the on-site laboratory service market, is estimated to drive the segment.
Based on product, the market is trifurcated into Whatman 903, Ahlstrom 226, and FTA. Whatman 903 is predicted to generate nearly 41.7% of the specimen collection cards market share in 2025. This is attributed to its compatibility with a wide range of analytes, proven performance in clinical workflows, and widespread regulatory acceptance. Whatman 903 paper is made from high-purity cotton linters and provides minimal analyte interference, consistent sample spreading, and uniform absorption. Due to its validated performance in PCR amplification and RNA extraction, several public health ministries in Africa and the UNICEF had placed bulk orders for Whatman 903 cards in 2023 for HIV Early Infant Diagnosis (EID) programs. Its reliability in field diagnostics was further strengthened as a study published in BMC Infectious Diseases the same year reported that Whatman 903-based DBS exhibited over 98% concordance with plasma-based testing for HIV viral load.
The FTA segment, on the other hand, is poised to rise at a considerable CAGR from 2025 to 2032. This is due to their robust nucleic acid preservation capabilities required for infectious disease, forensic, and genetic applications. FTA cards are chemically treated to lyse cells, denature proteins, and protect DNA and RNA from microbial growth as well as degradation. This makes them ideal for use in low-resource and field-based settings.
A 2023 study published by Frontiers in Genetics found that DNA stored on FTA cards remained amplifiable even after five years of ambient storage. It emphasized the long-term stability of these cards for longitudinal and bio-banking studies. The FTA buccal collection kits market is further gaining impetus due to their ease of non-invasive DNA collection in population-scale genetic screening and forensic identification.
In 2025, North America is estimated to account for a share of approximately 35.4%. It will likely be propelled by the boom of at-home sample collection services, rapid forensic developments, and the integration of decentralized diagnostics. The U.S. specimen collection cards market is predicted to outpace Canada through 2032 as the Centers for Disease Control and Prevention (CDC) focuses on extending DBS cards for infectious disease monitoring and newborn screening.
With more than 4 Mn newborns tested each year, DBS cards are currently used for newborn screening in all 50 U.S. states, solidifying their position in early disease diagnosis, says the Association of Public Health Laboratories (APHL). This trend contributes significantly to the newborn metabolic screening market, where the demand for minimally invasive yet reliable sample collection solutions continues to skyrocket. The U.S. is also witnessing a sharp rise in law enforcement and forensic applications. The U.S. Department of Justice extended its DNA collection programs in 2023 by using FTA buccal collection kits for detainees and arrestees. This has gradually led to a surge in the volume of non-invasive sample collection, thereby bolstering demand for chemically treated cards.
In the Middle East, the adoption of specimen collection cards is expected to be pushed by surging newborn screening programs. The Saudi Newborn Screening Program, which was rolled out by the Ministry of Health, now includes more than 18 disorders, including endocrine and metabolic conditions. The program has increased demand for DBS cards due to their stability and cost-effectiveness.
A 2022 study published in the International Journal of Neonatal Screening revealed that more than 95% of births in the Kingdom are screened by using DBS samples, making it a key program in the region.
Across sub-Saharan Africa, the use of DBS cards is skyrocketing in hepatitis and HIV surveillance, significantly transforming the HIV-AIDS testing market. As per a 2023 report by UNAIDS, countries such as Uganda, Kenya, and Nigeria are striving to scale up DBS-based viral load monitoring to reach populations in remote areas. This is because these cards help bypass the requirement for cold-chain logistics and enable delayed transportation without sample degradation. In Uganda alone, for example, more than 60% of viral load tests in rural districts are now conducted using DBS samples.
In Asia Pacific, the market growth will likely be spurred by the urgent requirement for decentralized diagnostics in underserved and rural areas. Countries such as China and India are at the forefront of this growth, embracing DBS cards for large-scale disease screening programs. India’s Rashtriya Bal Swasthya Karyakram (RBSK) has already extended newborn screening in various states using DBS cards to detect congenital disorders in early infancy. It aims to screen more than 270 Mn children under the National Health Mission.
China is currently using specimen collection cards mainly for infectious disease and pharmacogenomic research. The Chinese Center for Disease Control and Prevention recently pointed to the successful deployment of DBS cards in remote provinces, including Tibet and Yunnan for syphilis and hepatitis B testing. It found reduced sample rejection rates and high reach owing to ambient sample stability.
In South Korea and Japan, specimen collection cards are used for high-tech metabolomic and genomic studies. Whatman 903 and FTA are extensively being used by university-led cohort studies and biobanks for DNA extraction and storage. This rising adoption is closely linked to the DNA/RNA extraction machine market, which is gaining traction as research laboratories seek equipment compatible with dried samples.
The specimen collection cards market houses various niche biotechnology companies and well-established diagnostics firms. Leading players are focused on conducting in-depth research and development on innovative biosample preservation and collection technologies. They are striving to offer high-integrity sample preservation for RNA, DNA, and other analytes under non-refrigerated conditions, which is important for remote healthcare settings.
A few companies are strengthening their position by investing in molecular stability solutions, filter paper technology, and collaborations with public health programs. Emerging companies are aiming to provide application-specific or customizable cards designed for infectious disease surveillance, forensic analysis, or newborn screening. They are focusing on features, including multi-analyte sampling capacity and colorimetric indicators, to cater to varying requirements.
The market is projected to reach US$ 477.0 Mn in 2025.
Increasing demand for sample storage without cold chain infrastructure and the ongoing expansion of newborn screening programs are the key market drivers.
The market is poised to witness a CAGR of 5.0% from 2025 to 2032.
Surging requirement for forensic toxicology testing and increasing popularity of at-home testing kits are the key market opportunities.
QIAGEN N.V., PerkinElmer Inc., and Danaher Corporation are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Specimen Type
By Material
By Product
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author