- Executive Summary
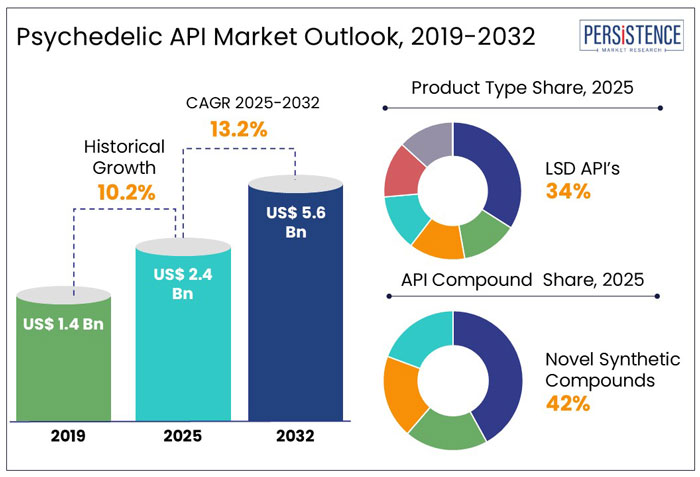
- Global Psychedelic API Market Snapshot, 2025 and 2032
- Market Opportunity Assessment, 2025 - 2032, US$ Mn
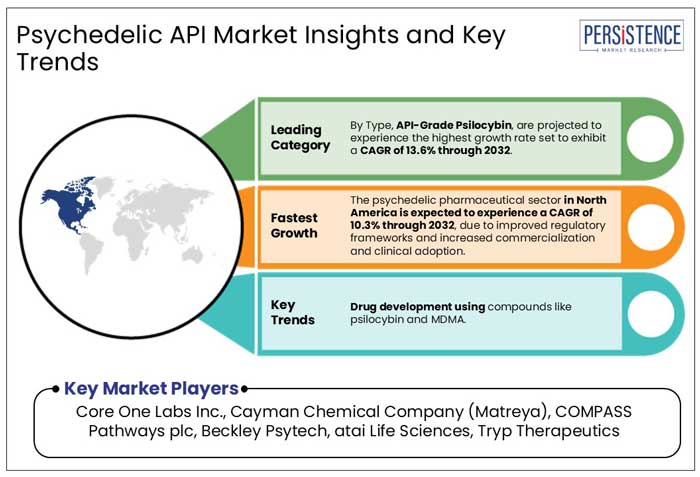
- Key Market Trends
- Future Market Projections
- Premium Market Insights
- Industry Developments and Key Market Events
- PMR Analysis and Recommendations
- Market Overviews
- Market Scope and Definition
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Challenges
- Key Trends
- Macro-Economic Factors
- Global Sectorial Outlook
- Global GDP Growth Outlook
- Global Healthcare Spending Outlook
- COVID-19 Impact Analysis
- Forecast Factors - Relevance and Impact
- Value Added Insights
- Drug Adoption Analysis
- Value Chain Analysis
- List of Sourcer/Marketplaces
- Retail
- E-Commerce
- List of Indication (Industry)
- List of Sourcer/Marketplaces
- Key Deals and Mergers
- PESTLE Analysis
- Porter’s Five Force Analysis
- Global Psychedelic API Market Outlook
- Key Highlights
- Market Size (US$ Mn) and Y-o-Y Growth
- Absolute $ Opportunity
- Market Size (US$ Mn) Analysis and Forecast
- Historical Market Size (US$ Mn) Analysis, 2019-2023
- Current Market Size (US$ Mn) Analysis and Forecast, 2024-2032
- Global Psychedelic API Market Outlook: Product Type
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Product Type, 2019 - 2023
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Market Attractiveness Analysis: Product Type
- Global Psychedelic API Market Outlook: API Compound
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By API Compound, 2019 - 2023
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Market Attractiveness Analysis: API Compound
- Global Psychedelic API Market Outlook: Source
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Source, 2019 - 2023
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Market Attractiveness Analysis: Source
- Global Psychedelic API Market Outlook: Grade
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Grade, 2019 - 2023
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Market Attractiveness Analysis: Grade
- Global Psychedelic API Market Outlook: Application
- Introduction / Key Findings
- Historical Market Size (US$ Mn) Analysis, By Application, 2019 - 2023
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis: Application
- Key Highlights
- Global Psychedelic API Market Outlook: Region
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Region, 2019 - 2023
- Current Market Size (US$ Mn) Analysis and Forecast, By Region, 2024 - 2032
- North America
- Europe
- East Asia
- South Asia and Oceania
- Latin America
- Middle East & Africa
- Market Attractiveness Analysis: Region
- North America Psychedelic API Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019 - 2023
- By Country
- By Product Type
- By API Compound
- By Source
- By Grade
- By Application
- Current Market Size (US$ Mn) Analysis and Forecast, By Country, 2024 - 2032
- U.S.
- Canada
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis
- Europe Psychedelic API Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019 - 2023
- By Country
- By Product Type
- By API Compound
- By Source
- By Grade
- By Application
- Current Market Size (US$ Mn) Analysis and Forecast, By Country, 2024 - 2032
- Germany
- France
- U.K.
- Italy
- Spain
- Russia
- Türkiye
- Rest of Europe
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis
- East Asia Psychedelic API Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019 - 2023
- By Country
- By Product Type
- By API Compound
- By Source
- By Grade
- By Application
- Current Market Size (US$ Mn) Analysis and Forecast, By Country, 2024 - 2032
- China
- Japan
- South Korea
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis
- South Asia and Oceania Psychedelic API Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019 - 2023
- By Country
- By Product Type
- By API Compound
- By Source
- By Grade
- By Application
- Current Market Size (US$ Mn) Analysis and Forecast, By Country, 2024 - 2032
- India
- Southeast Asia
- ANZ
- Rest of South Asia & Oceania
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis
- Latin America Psychedelic API Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019 - 2023
- By Country
- By Product Type
- By API Compound
- By Source
- By Grade
- By Application
- Current Market Size (US$ Mn) Analysis and Forecast, By Country, 2024 - 2032
- Brazil
- Mexico
- Rest of Latin America
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis
- Middle East & Africa Psychedelic API Market Outlook
- Key Highlights
- Historical Market Size (US$ Mn) Analysis, By Market, 2019 - 2023
- By Country
- By Product Type
- By API Compound
- By Source
- By Grade
- By Application
- Current Market Size (US$ Mn) Analysis and Forecast, By Country, 2024 - 2032
- GCC Countries
- Egypt
- South Africa
- Northern Africa
- Rest of Middle East & Africa
- Current Market Size (US$ Mn) Analysis and Forecast, By Product Type, 2024 - 2032
- API-Grade Psilocybin
- API-Grade DMT
- LSD API
- MDMA API
- Ketamine API
- Others
- Current Market Size (US$ Mn) Analysis and Forecast, By API Compound, 2024 - 2032
- Active Plant Components
- Novel Synthetic Compounds
- Metabolites
- Isotopically Labelled Standards
- Current Market Size (US$ Mn) Analysis and Forecast, By Source, 2024 - 2032
- Natural
- Synthetic
- Bio-Synthetic
- Current Market Size (US$ Mn) Analysis and Forecast, By Grade, 2024 - 2032
- GMP
- Non-GMP
- Current Market Size (US$ Mn) Analysis and Forecast, By Application, 2024 - 2032
- Clinical
- Research
- Market Attractiveness Analysis
- Competition Landscape
- Market Share Analysis, 2024
- Market Structure
- Competition Intensity Mapping by Market
- Competition Dashboard
- Company Profiles (Details - Overview, Financials, Strategy, Recent Developments)
- Core One Labs Inc.
- Overview
- Segments and Products
- Key Financials
- Market Developments
- Market Strategy
- Cayman Chemical Company (Matreya)
- COMPASS Pathways plc
- Beckley Psytech
- Atai Life Sciences
- Tryp Therapeutics
- Filament Health
- Ceruvia Lifesciences, LLC
- BetterLife Pharma
- Psygen Pharmaceutical
- Mindset Pharma
- Core One Labs Inc.
- Appendix
- Research Methodology
- Research Assumptions
- Acronyms and Abbreviations

Loading page data
Please wait a moment