ID: PMRREP4769| 246 Pages | 21 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

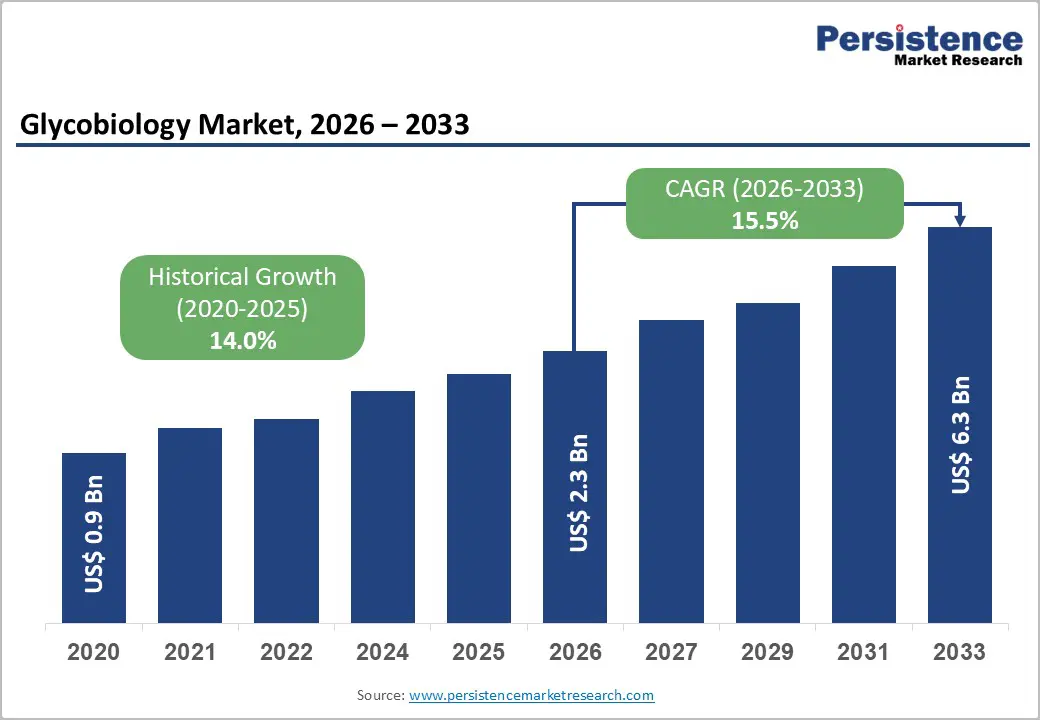
The global glycobiology market is estimated to grow from US$ 2.3 Bn in 2026 to US$ 6.3 Bn by 2033. The market is projected to record a CAGR of 15.5% during the forecast period from 2026 to 2033.
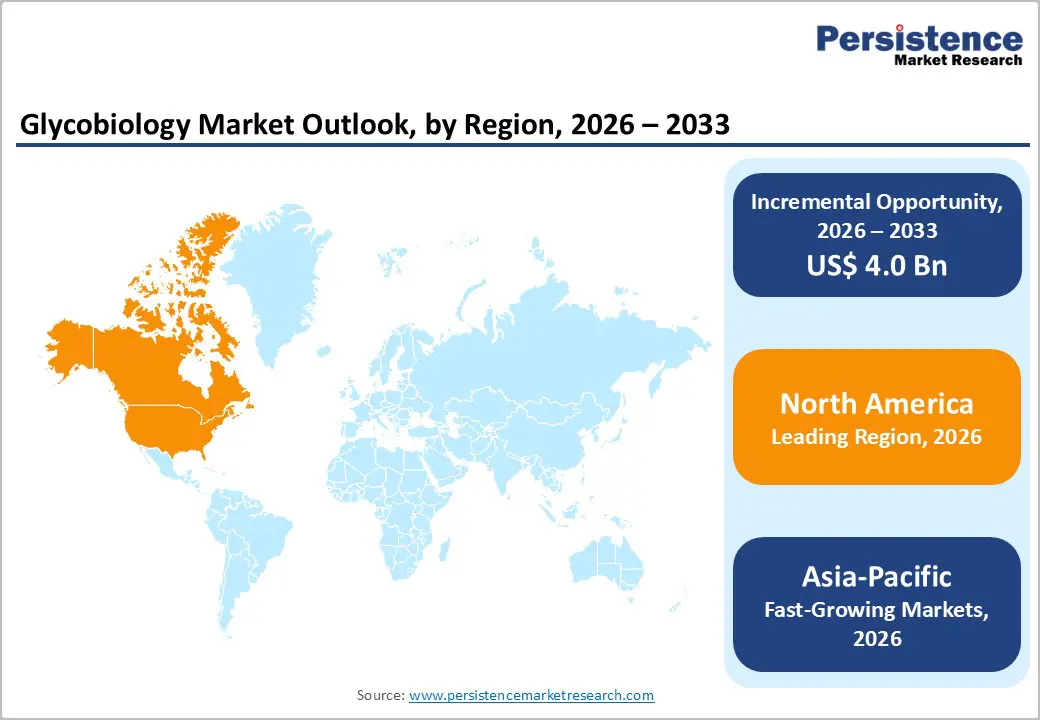
The global glycobiology industry is expanding steadily, driven by the adoption of digital healthcare, telehealth, and advanced analytics. North America leads due to robust infrastructure, strict regulations, and high laboratory standards. Asia-Pacific is the fastest-growing region, supported by expanding healthcare facilities, government digital initiatives, rising patient awareness, and growing investments in diagnostic software, services, and interoperable testing solutions.
| Global Market Attributes | Key Insights |
|---|---|
| Glycobiology Market Size (2026E) | US$ 2.3 Bn |
| Market Value Forecast (2033F) | US$ 6.3 Bn |
| Projected Growth (CAGR 2026 to 2033) | 15.5% |
| Historical Market Growth (CAGR 2020 to 2025) | 14.0% |
Driver: Increasing demand for early disease diagnosis and monitoring
The glycobiology market is driven by the increasing global emphasis on early disease detection and monitoring, enabling timely intervention and better health outcomes. According to the World Health Organization (WHO), diagnostics influence approximately 70% of healthcare decisions, underscoring the critical role of early and accurate detection in clinical care. Early identification of noncommunicable diseases like cancer, diabetes, and cardiovascular conditions through advanced biomarkers and surveillance systems supports prevention and management strategies and reduces long-term care costs. Enhanced monitoring systems and a stronger focus on disease surveillance by public health bodies further emphasize the importance of early diagnosis to mitigate disease progression and improve survival rates.
Glycobiology contributes significantly to early disease diagnosis by identifying glycan-based biomarkers that can indicate pathological changes before symptoms appear. Research shows that aberrant glycosylation patterns are associated with cancer progression and can be detected in blood, offering non-invasive diagnostic potential. For pancreatic cancer, N-glycan signatures demonstrated diagnostic sensitivity of nearly 90% for early-stage detection in clinical studies, highlighting the promise of glycan analysis for early identification. These biomarkers provide insight into underlying disease mechanisms and, when integrated with clinical monitoring, support more precise tracking of disease onset, progression, and response to therapy.
Restraints: Complexity of glycobiology workflows and specialized technical requirements
A key restraint in the glycobiology market is the complexity of workflows and the expertise required to accurately study glycans. Glycans are structurally diverse and highly branched, making their analysis far more complex than that of proteins or nucleic acids. Techniques such as mass spectrometry, high-performance liquid chromatography, and capillary electrophoresis are commonly used to resolve glycan structures. In addition, interpreting results demands knowledge of bioinformatics and glycan databases. Many clinical and research laboratories face challenges implementing these protocols due to their intricacy, length, and need for highly trained personnel, which can slow adoption of glycobiology-based diagnostics and therapeutics.
The specialized technical requirements in glycobiology further restrict broader use. Glycan profiling requires precise instrumentation, controlled sample preparation, and extensive computational analysis to differentiate isomers and branching patterns. Standardizing protocols is difficult because minor variations in reagents, instruments, or workflows can significantly affect results. As a result, only laboratories with skilled staff in analytical chemistry, molecular biology, and bioinformatics can reliably perform advanced glycan studies. This talent scarcity limits accessibility and slows translation from research to clinical practice. Even institutions with resources often face delays due to training needs, long experimental timelines, and intricate quality control requirements.
Opportunity: Development of point-of-care metabolic and glycan testing
The development of point-of-care (POC) metabolic and glycan testing presents a significant opportunity in the glycobiology market by enabling rapid, decentralized diagnostic insights at or near patient care sites. POC technologies deliver results quickly, often within minutes, allowing clinicians to make timely treatment decisions without relying on centralized laboratory infrastructure, which traditionally requires sample transport and delayed turnaround. Rapid POC results improve clinical and economic outcomes by reducing delays in therapy and facilitating earlier interventions for conditions such as diabetes and metabolic disorders. These attributes align with global healthcare priorities for accessible diagnostics, especially where conventional lab access is limited.
Evidence from clinical settings shows that POC diagnostics, such as hemoglobin A1c tests, yield highly correlated results with traditional lab assays, demonstrating their feasibility in community and decentralized settings. For example, POC hemoglobin A1c testing demonstrated a strong correlation (0.85) with laboratory results, indicating reliable assessment of a metabolic marker outside centralized labs and high participant satisfaction with community testing initiatives. Additionally, POC testing platforms are being adopted globally for a range of biochemical markers, underscoring the role of rapid, on-site diagnostics in patient management and monitoring. These developments suggest that expanding POC metabolic and glycan testing could strengthen early disease detection, improve monitoring, and broaden access to essential diagnostics.
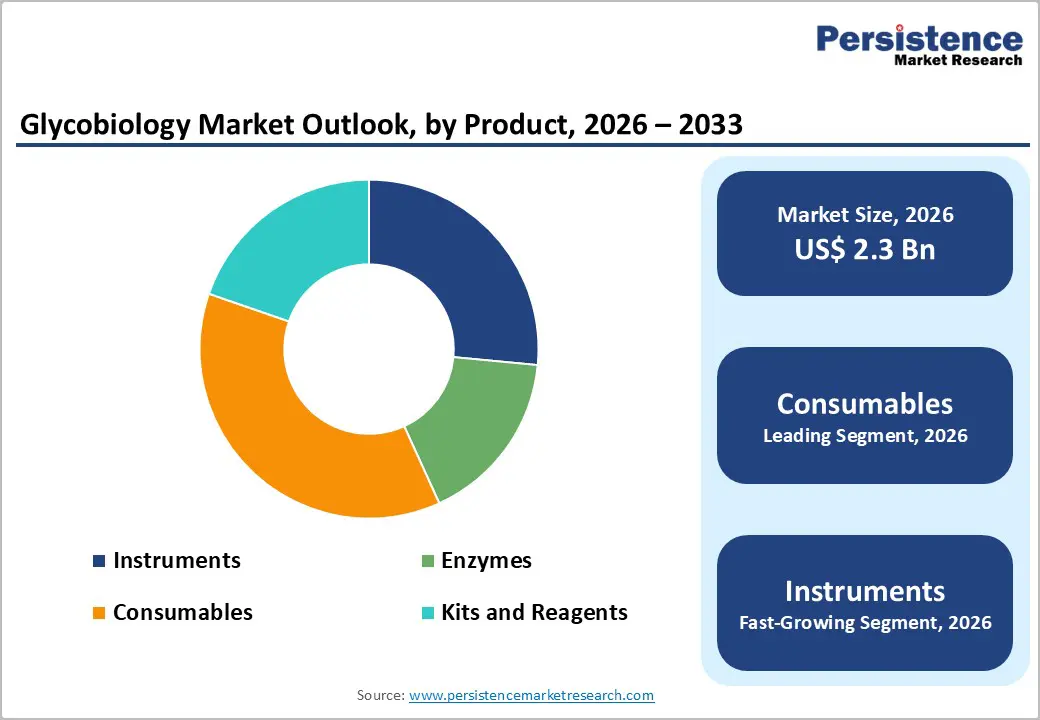
By Product, Consumables Dominates the Glycobiology Market
Consumables occupies 37.1% share of the global market in 2025, because they are essential for every step of glycan analysis and diagnostic workflows. Glycobiology involves complex processes such as glycan release, labeling, derivatization, separation, and detection, all of which require high-quality reagents, enzymes, buffers, and assay kits. Unlike instruments, consumables are single-use and must be replenished for each experiment or test, creating recurring demand. For example, the NIH Glycomic program and interlaboratory studies conducted by NIST rely on standardized consumables to ensure accuracy, reproducibility, and comparability of glycan data across multiple labs. Additionally, consumables support emerging techniques like lectin microarrays, enzymatic profiling, and high-throughput analyses, making them indispensable for research, clinical diagnostics, and biomarker discovery in glycobiology.
By Application, Drug Discovery and Development dominates due to glycans’ critical role in therapeutic design, protein function, and biologics development
By application, drug discovery and development dominate the glycobiology market because glycobiology is central to the design and optimization of modern therapeutics, particularly biologics and targeted treatments. Glycans, the carbohydrate portions of glycoproteins and glycolipids, are crucial for protein folding, stability, immune recognition, cell signaling, and drug–receptor interactions, making them essential targets and quality attributes in drug design and evaluation. For example, more than half of approved protein therapeutics, such as monoclonal antibodies, are glycoproteins, and their glycosylation patterns directly influence efficacy, stability, and half-life.
Government-linked research centers, such as the National Center for Functional Glycomics, focus on developing glycan analysis technologies to support drug discovery and biological understanding, reflecting public investment in glycoscience for therapeutics. This foundational role in creating safe, effective, and stable drugs explains why drug discovery and development remain the leading application segment in the field of glycobiology.
North America Glycobiology Market Trends
North America dominates the glycobiology market with a 44.4% share in 2025, thanks to robust research infrastructure, strong public funding, and a vibrant biotechnology and pharmaceutical ecosystem. Federal agencies such as the U.S. National Institutes of Health (NIH), the National Science Foundation (NSF), and others actively support glycoscience research, including specialized centers such as the National Center for Functional Glycomics, which advances glycan analysis technologies and training.
The U.S., the largest contributor within the region, benefits from concentrated academic–industry collaborations and high research output in glycobiology and related fields. This environment accelerates innovation in biomarkers, therapeutics, and diagnostics that rely on glycan characterization, reinforcing North America’s leadership in research, clinical applications, and commercialization of glycobiology solutions.
Europe Glycobiology Market Trends
Europe is a key region in the glycobiology market due to its advanced research infrastructure, collaborative scientific networks, and strong healthcare investment. Programs like the European Union’s Horizon Europe fund multidisciplinary research, including glycoscience, promoting innovation, biomarker discovery, and translational studies across member states. Public health systems in Europe collectively spend around 10% of GDP on healthcare, reflecting significant support for diagnostic research and clinical applications.
European institutions such as ELIXIR and the European Glycoscience Community facilitate data sharing, training, and collaboration between academia, industry, and clinics. This integration strengthens glycobiology research, accelerates biomarker and therapeutic development, and enhances Europe’s position as a hub for innovation in disease diagnostics and drug development.
Asia-Pacific Glycobiology Market Trends
Asia Pacific is the fastest-growing region in the glycobiology market, driven by rapid demographic shifts, rising chronic disease prevalence, and expanding healthcare infrastructure. The region’s aging population is growing rapidly; by 2050, nearly 22% of the Asia Pacific’s population will be over 65, increasing demand for diagnostics and monitoring of age-related metabolic and glycan-linked conditions. Additionally, non-communicable diseases such as diabetes and cardiovascular disorders affect millions, with the World Health Organization reporting over 276 million adults with diabetes in South-East Asia, highlighting the need for early detection and monitoring.
Governments are also investing in digitalization of healthcare and telehealth, improving accessibility and enabling point-of-care diagnostics. High smartphone and internet penetration, exceeding 70%, supports remote monitoring and patient-centric care. This combination of demographic growth, disease burden, and technological adoption drives strong demand for glycobiology testing and diagnostic solutions across the region.
Leading glycobiology market companies focus on advanced assays, scalable platforms, and regulatory compliance. Investments in AI-enabled analysis, high-throughput testing, and process optimization enhance accuracy and reproducibility. Collaborations with research institutions, standardized protocols, quality control, and integrated supply chains drive innovation and support widespread adoption in clinical diagnostics, preventive metabolic care, and biomarker-based research.
Key Industry Developments:
The global glycobiology market is projected to be valued at US$ 2.3 Bn in 2026.
Rising chronic diseases, demand for early diagnosis, advanced assays, digital health integration, and personalized therapies.
The global glycobiology market is poised to witness a CAGR of 15.5% between 2026 and 2033.
Opportunities include point-of-care testing, AI-enabled diagnostics, multiplex assays, personalized medicine, telehealth integration, and emerging markets.
Glycodiag, Tracxn Technologies Limited, Agilent Technologies, Inc., Takara Bio Inc., Bio-Techne, Bruker Corporation.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author