ID: PMRREP34923| 184 Pages | 4 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

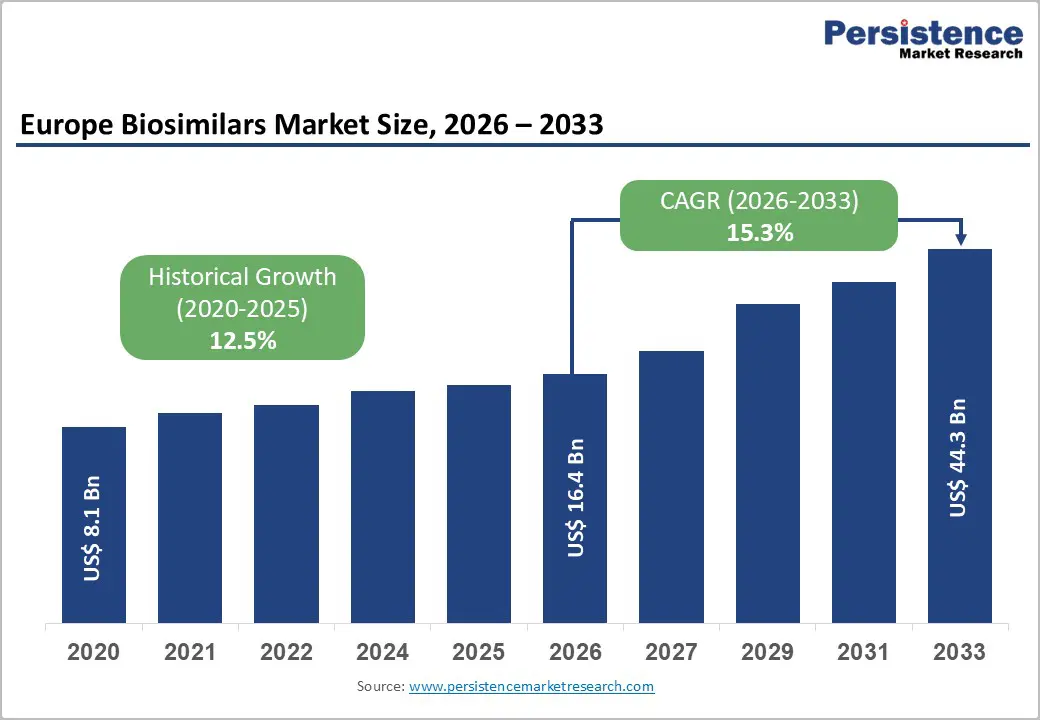
Europe biosimilars market size is expected to be valued at US$ 16.4 billion in 2026 and projected to reach US$ 44.3 billion by 2033, growing at a CAGR of 15.3% between 2026 and 2033.
Affordable alternatives to costly biologic therapies are rapidly transforming the biosimilars market here. Driven by increasing demand for cost-effective treatments, biosimilars are gaining prominence across oncology, immunology, and rheumatology. Patent expiries of several blockbuster biologics have created opportunities for biosimilars to deliver substantial savings while maintaining comparable safety and efficacy. Strong regulatory support from the European Medicines Agency (EMA) has accelerated approvals, enabling wider adoption and integration into healthcare systems.
The adoption of biosimilars has already generated cumulative savings exceeding €50 billion since 2006, with €10 billion saved in 2023 alone. Trends such as value-based healthcare, improved patient access, and expanded hospital pharmacy distribution are further fueling growth. With ongoing R&D investment and increasing therapeutic options for chronic diseases, the Europe biosimilars market is poised for sustained expansion through 2033.
| Key Insights | Details |
|---|---|
| Biosimilars Market Size (2026E) | US$ 16.4 Bn |
| Market Value Forecast (2033F) | US$ 44.3 Bn |
| Projected Growth (CAGR 2026 to 2033) | 15.3% |
| Historical Market Growth (CAGR 2020 to 2024) | 12.5% |
Many countries in Europe have recognized the importance of biosimilars in controlling healthcare costs, leading to favourable reimbursement policies that ensure these therapies are included in public health programs. This has encouraged healthcare providers to prescribe biosimilars frequently, knowing that they are financially supported.
Pricing strategies in several regions have been designed to make biosimilars affordable, allowing them to compete effectively with originator biologics. These policies not only drive market adoption but also enhance patient access to high-quality, cost-effective treatment options across Europe. Wider reimbursement and pricing approvals across Europe have significantly contributed to the Europe biosimilars market growth.
European Medicines Agency (EMA) plays a central role by providing clear regulatory pathways for biosimilars, ensuring they meet rigorous safety, efficacy, and quality standards. This streamlined approval process has accelerated the market entry of biosimilars, fostering competition with originator biologics and helping reduce overall healthcare costs. As of 2024, 106 biosimilars have been approved in Europe, with 17 being withdrawn post approval, while 89 still approved for commercial use in European nation. (Generics and Biosimilars Initiative (GaBI),
Many European governments have implemented policies to promote biosimilars, supporting their adoption in various therapeutic areas. These regulatory frameworks not only build market confidence but also improve the accessibility of biosimilars to a wider patient population.
Intellectual property (IP) and patent litigation represent significant challenges for the Europe biosimilars market. Originator biologics often have complex patent portfolios, which can delay the entry of biosimilars due to legal disputes. Patent holders may extend their exclusivity through "evergreening" practices, where they file new patents for modified versions of biologics, thereby prolonging market monopolies.
Biosimilar manufacturers face high legal costs and risks associated with patent challenges, often leading to delays in market entry. This uncertainty impacts investment decisions and slows the availability of affordable biosimilar alternatives. As patent litigation continues, it can hinder competition and the broad adoption of biosimilars in Europe.
Adoption of biosimilars in some countries of Europe is quite slow, influenced by several factors including conservative prescribing practices, a lack of awareness among healthcare professionals, and varying levels of regulatory confidence. Physicians are hesitant to switch from established biologics to biosimilars in certain regions due to concerns about their clinical effectiveness, despite rigorous regulatory approvals. In others, the price differences between biologics and biosimilars being minimal because of no pricing rules for biosimilars also impact the biosimilar uptake.
Cultural and healthcare system differences can result in uneven uptake, with some countries slower to embrace biosimilars due to financial, logistical, or educational barriers. This delayed adoption limits the potential market growth of biosimilars, preventing patients in these regions from benefiting from affordable treatment options.
Opportunity – Potential for Biosimilars in Biosimilar-Reference Biologic Switching
The potential for biosimilars in biosimilar-reference biologic switching presents a significant opportunity in Europe biosimilars market. As healthcare systems increasingly prioritize cost-effective treatments, the ability to switch patients from originator biologics to biosimilars can further reduce healthcare expenses. This practice is particularly relevant in chronic disease management, where long-term treatment is required. Furthermore, total of 10 biological medicines is expected to lose intellectual property (IP) protection in the next decade providing loss of exclusivity opportunities of nearly €30 Bn between 2030 and 2032.
Regulatory agencies, such as the EMA support this concept by approving biosimilar interchangeability in many cases, allowing patients to transition smoothly between treatments. As physician confidence in biosimilars grows, switching programs could lead to broad adoption and further price competition, benefiting both patients and healthcare providers.
The expansion of biosimilars into non-oncology therapeutic areas presents a significant growth opportunity in the Europe biosimilars market. While oncology has traditionally been the dominant focus, biosimilars are increasingly being developed for conditions such as autoimmune diseases, diabetes, and inflammatory disorders. These areas represent large patient populations where biologic treatments are often expensive.
As healthcare systems strive for cost containment, biosimilars offer an affordable alternative, promoting wide access to life-saving therapies. Regulatory support and increasing physician confidence in biosimilar efficacy are also driving this expansion, potentially transforming the treatment landscape for chronic and complex diseases across Europe.
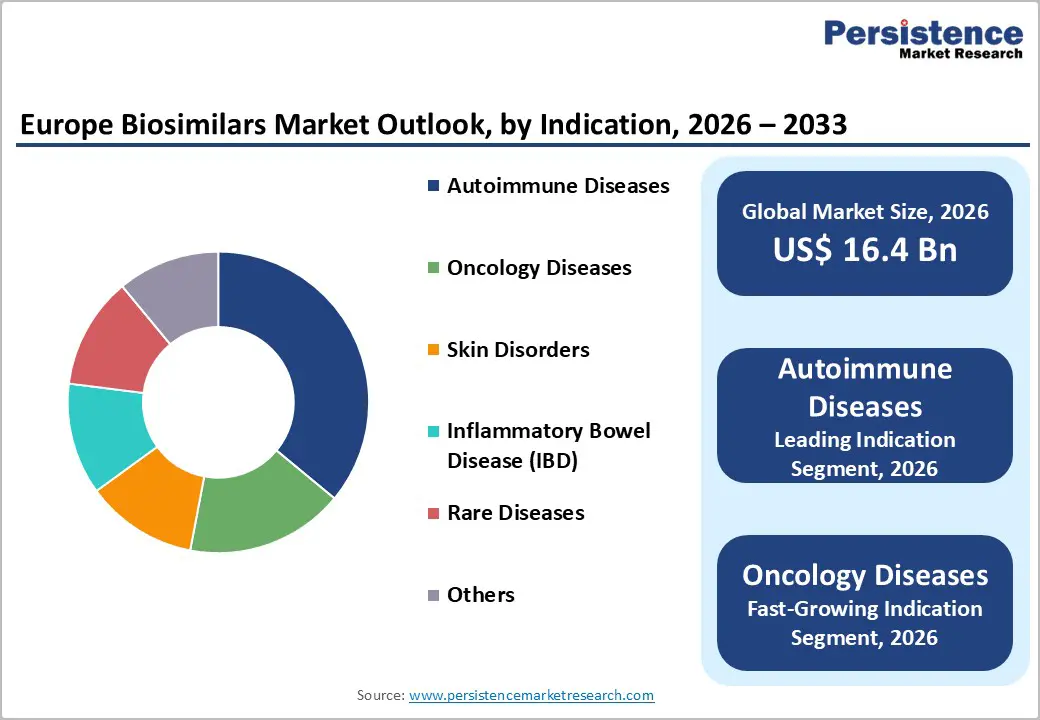
By indication, autoimmune diseases dominate the Europe biosimilars market, accounting for approximately 36% market share. This dominance is driven by the high prevalence of chronic conditions such as rheumatoid arthritis, psoriasis, Crohn’s disease, and ankylosing spondylitis across Europe. Long-term treatment requirements, combined with the high cost of originator biologics, have accelerated the adoption of biosimilars in this segment. Strong physician familiarity, well-established clinical evidence, and favorable reimbursement policies further support widespread biosimilar use in autoimmune therapies, particularly within hospital and specialty care settings.
Meanwhile, oncology diseases represent the fastest-growing segment, projected to register a CAGR of around 13.7% during the forecast period. Rapid growth is supported by rising cancer incidence, increasing pressure on healthcare budgets, and the expiry of patents for blockbuster oncology biologics. Oncology biosimilars provide substantial cost savings while maintaining comparable safety and efficacy, improving patient access to life-saving treatments. Supportive EMA regulations and expanding reimbursement coverage are expected to further accelerate biosimilar uptake in oncology across Europe.
Based on distribution channel, hospital pharmacies represent the fastest-growing segment in the Europe biosimilars market, projected to register a CAGR of approximately 14.2% between 2026 and 2033. This growth is primarily attributed to the rising use of biosimilars in oncology, immunology, and other hospital-administered therapies. Hospitals remain the primary point of care for complex biologic treatments, making hospital pharmacies central to biosimilar dispensing and treatment management.
Hospital pharmacies play a critical role in enabling cost savings by substituting high-cost originator biologics with approved biosimilars, while maintaining comparable clinical outcomes. Their involvement supports standardized treatment protocols, close patient monitoring, and improved adherence to regulatory and reimbursement guidelines.
Additionally, European healthcare systems increasingly emphasize budget optimization, centralized procurement, and value-based care models, further strengthening hospital pharmacy adoption. The growing availability of biosimilars for chronic and specialty indications, combined with strong physician confidence and institutional support, continues to reinforce the expanding importance of hospital pharmacies in biosimilar distribution across Europe.
Europe biosimilars market features a dynamic and rapidly evolving competitive landscape, dominated by a mix of established pharmaceutical giants and specialized biosimilar manufacturers. Leading players such as Sandoz, Amgen, Samsung Biologics, Biogen, and Celltrion offer biosimilars across key therapeutic areas, including oncology, immunology, and endocrinology. These companies leverage robust research and development capabilities, regulatory expertise, and strategic collaborations with healthcare providers to strengthen their market presence and expand product portfolios.
Market competition is intensifying as new entrants emerge, attracted by the rising demand for affordable biologics. Key factors influencing this environment include pricing pressures, patent disputes, and the need to differentiate products based on quality, safety, and efficacy. Companies that innovate while ensuring regulatory compliance are poised to gain a competitive advantage in this growing market.
Europe biosimilars market is projected to be valued at US$ 16.4 Bn in 2026.
Rising biologic treatment costs, patent expirations, strong healthcare policies, and growing physician & patient acceptance of biosimilars.
Europe is expected to witness a CAGR of 15.3% between 2026 and 2033.
Expansion in oncology & autoimmune therapies, emerging markets, cost-saving initiatives, partnerships, and development of next-generation biosimilars.
The major players in Europe biosimilars market include Fresenius Kabi, Biogen Inc., Celltrion, Inc., Pfizer Inc., and Amgen Inc.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn and Volume (if Available) |
| Country Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Drug
By Drug Class
By Indication
By Distribution Channel
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author