ID: PMRREP25045| 199 Pages | 10 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

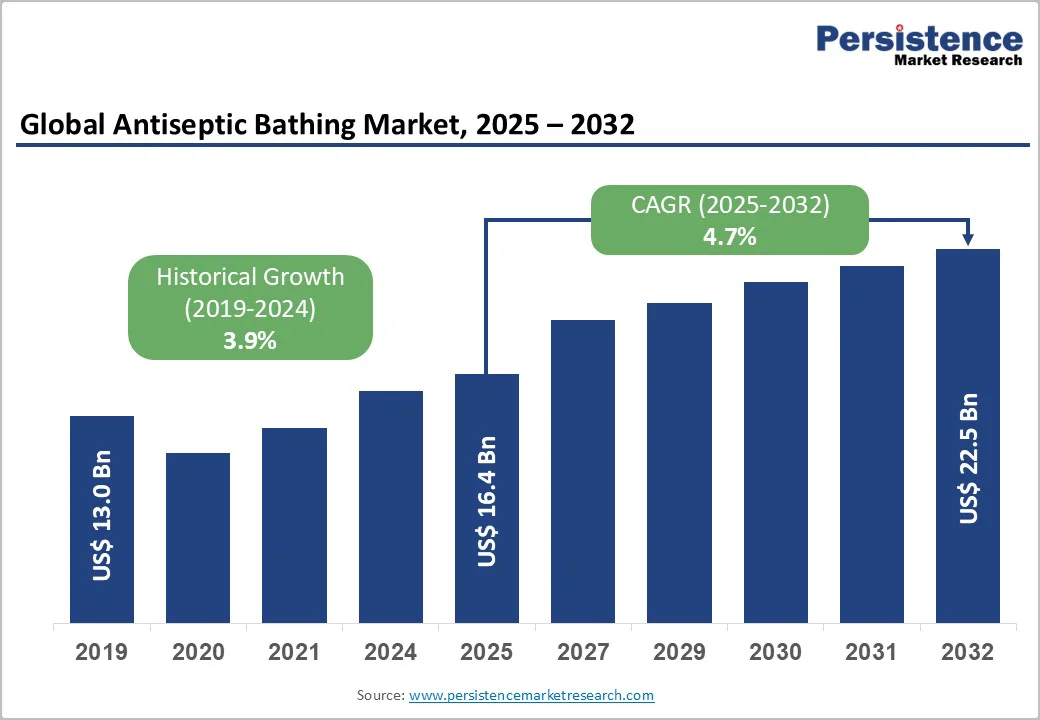
The global antiseptic bathing market size is valued at US$16.4 billion in 2025 and is projected to reach US$22.5 billion by 2032, growing at a CAGR of 4.7% between 2025 and 2032. As healthcare facilities emphasize infection prevention and patient safety, there has been a huge need for antiseptic bathing accessories.
Rising incidences of hospital-acquired infections (HAIs), expanding surgical volumes, and increasing ICU capacity drive the adoption of antiseptic solutions across hospitals, long-term care facilities, and high-risk wards.
Key products, such as chlorhexidine gluconate (CHG) solutions and octenidine-based formulations, are gaining prominence due to proven efficacy in reducing central line-associated bloodstream infections (CLABSIs), surgical site infections (SSIs), and other critical HAIs. Additionally, regulatory guidelines and growing awareness of bundled infection-control protocols further support market expansion globally.
| Key Insights | Details |
|---|---|
| Global Antiseptic Bathing Market Size (2025E) | US$16.4 Billion |
| Market Value Forecast (2032F) | US$22.5 Billion |
| Projected Growth (CAGR 2025 to 2032) | 4.7% |
| Historical Market Growth (CAGR 2019 to 2024) | 3.9% |
The global antiseptic bathing market is expanding as hospitals confront the rising burden of hospital-acquired infections (HAIs), which continue to increase morbidity, mortality, treatment costs, and inpatient stays.
Universal antiseptic bathing has gained prominence alongside standard precautions, particularly for ICU patients, where preventing healthcare-associated bloodstream infections (HABSIs), central line-associated bloodstream infections (CLABSIs), and acquisition of multidrug-resistant organisms (MDROs) remains a clinical priority.
In 2022, the Society for Healthcare Epidemiology of America (SHEA) designated daily chlorhexidine bathing for ICU patients over 2 months of age as an essential CLABSI-prevention practice, reinforcing its widespread use in critical care and for preoperative SSI prevention. However, growing concerns over chlorhexidine resistance and adverse reactions have accelerated interest in alternative agents.
A pivotal 2024 study analyzing 104,039 ICU episodes from 93,438 patients reported a 17% risk reduction in ICU-acquired primary bacteremia with octenidine-impregnated washcloths, particularly against coagulase-negative staphylococci (53%) and enterococci (17%). Although MDRO reduction was not observed, the findings highlight the value of octenidine in infection-prevention bundles.
Current SSI guidelines endorse alcohol-containing antiseptics, typically isopropanol combined with CHG or povidone-iodine, for surgical preparation, noting advantages such as reduced skin irritation and faster application. As ICUs expand globally and surgical volumes rise, the need for effective, well-tolerated antiseptic bathing solutions continues to strengthen market demand.
The global antiseptic bathing market faces growing restraint due to rising concerns over product safety, contamination risks, and vulnerability among high-risk patient groups. In August 2025, a major nationwide recall in the United States underscored these challenges when DermaRite Industries pulled four antiseptic and antimicrobial soaps after detecting contamination with Burkholderia cepacia complex (Bcc) a multidrug-resistant bacterium commonly found in soil and water.
Bcc poses severe infection risks and can lead to life-threatening sepsis, particularly in patients with open wounds, chronic illnesses, or weakened immunity. Although no infections were reported at the time, federal health agencies emphasized the immediate destruction of affected products to avert potential harm.
Such incidents heighten regulatory scrutiny and undermine clinical confidence in antiseptic bathing products, especially those used in hospitals, long-term care facilities, nursing homes, and home-based wound care. Contamination episodes also expose manufacturing gaps, prompting stricter quality-control expectations, higher compliance costs, and delays in product approvals.
Additionally, concerns about emerging antimicrobial resistance, allergic reactions, and variable product efficacy further challenge widespread adoption. Collectively, these safety-related barriers slow market penetration and reinforce the need for more robust manufacturing standards, transparent supply-chain monitoring, and validated antiseptic formulations to maintain trust and ensure patient protection.
The growing demand for barrier-breaking emollient (BBE) formulations, single-use pre-packaged pre-moistened bathing (PPMB) wipes, and alcohol-based hand rub (ABHR)-integrated bathing kits presents a significant opportunity in the antiseptic bathing market.
BBE formulations help reduce skin irritation and dryness, thereby improving patient comfort and compliance, which is critical in intensive-care and high-risk wards. Meanwhile, PPMB wipes deliver standardized dosing and convenience in critical care units and long-term care settings, supporting infection-control bundles efficiently.
ABHR-integrated kits further enhance hygiene outcomes: a 2022 review noted that ABHR systems, including emollients and chlorhexidine additives, improved hand-hygiene compliance and microbial kill rates in ICUs.
Combining ABHR with bathing products offers a bundled approach, enabling hospitals to streamline workflows, reduce device-associated infection risk and cater to stricter cleanliness standards. With rising emphasis on infection prevention and patient-safety protocols, this triad of enhanced formulations, convenient formats, and bundled solutions presents a significant opportunity in the antiseptic bathing category.
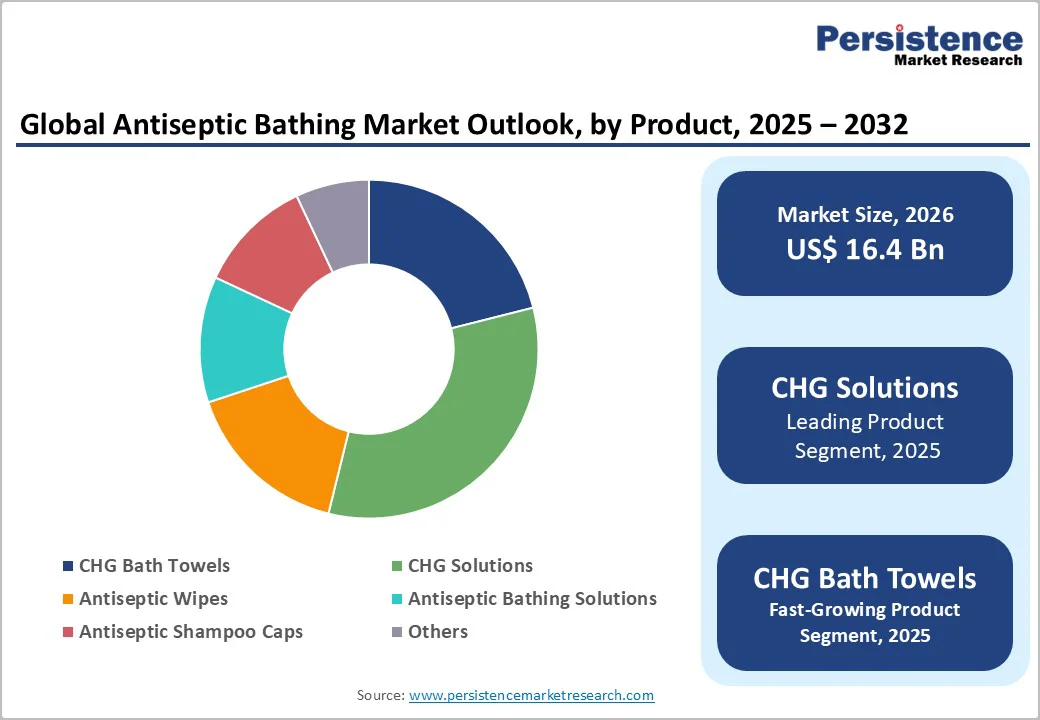
CHG (Chlorhexidine Gluconate) solutions are projected to capture a leading 32.7% share of the global antiseptic bathing market by 2025. Their dominance is driven by well-documented efficacy in reducing central line-associated bloodstream infections (CLABSIs), surgical site infections (SSIs), and other healthcare-associated infections (HAIs).
Daily CHG bathing is widely implemented in ICUs, long-term care facilities, and surgical wards, supported by clinical guidelines from SHEA and CDC. Increasing adoption across hospitals, rising critical-care capacity, and strong evidence from large-scale ICU studies further reinforce CHG solutions as the preferred choice for patient safety and infection prevention globally.
Surgical wards are projected to dominate the global antiseptic bathing market in 2025, accounting for nearly 38.4% of the total share. This leadership is fueled by the critical role of preoperative antiseptic bathing in reducing surgical site infections (SSIs) and improving postoperative outcomes.
Hospitals increasingly integrate antiseptic bathing into standard infection-prevention protocols for high-risk surgical patients. The combination of rising surgical volumes, adherence to international guidelines, and the need for standardized infection-control practices in operating theatres drives strong demand. This trend positions surgical wards as a major growth segment for antiseptic bathing products worldwide.
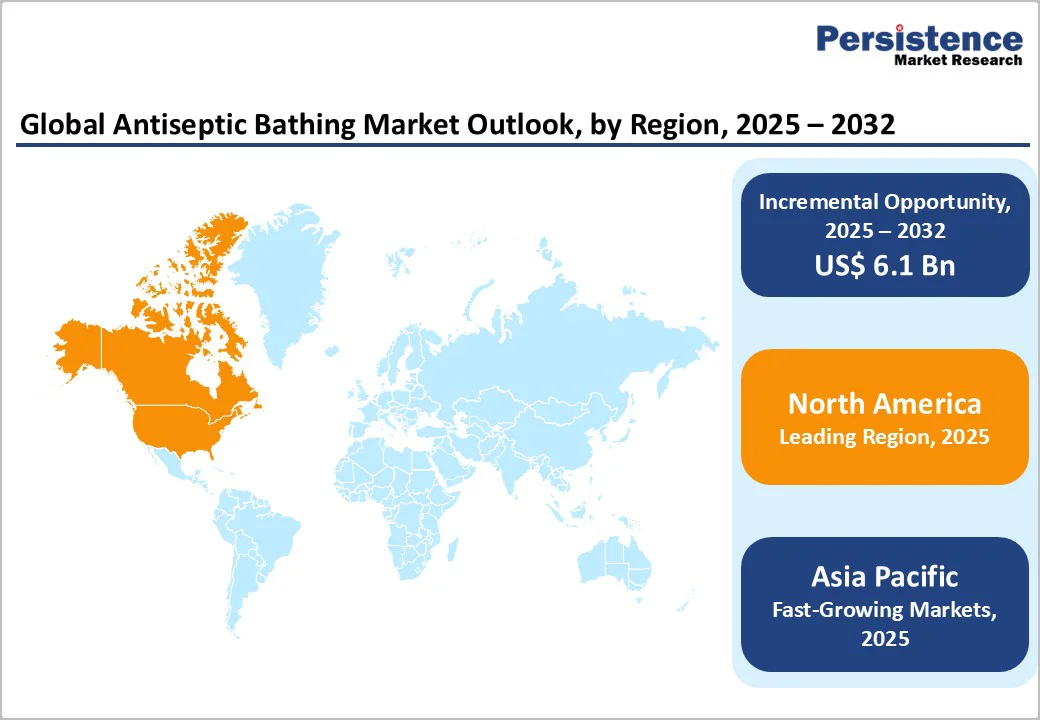
By 2025, North America is projected to command nearly 34.4% of the global antiseptic bathing market, supported by rising HAI (Healthcare-Associated Infection) prevalence, expanding surgical and ICU (Intensive Care Unit) volumes, and stricter infection-control mandates across hospitals and long-term care.
The 2023 National and State HAI Progress Report underscores this need, noting a 3% rise in SSI (Surgical Site Infection) combined SCIP (Surgical Care Improvement Project) procedures between 2022 and 2023 across ACHs (Acute Care Hospitals), CAHs (Critical Access Hospitals), IRFs (Inpatient Rehabilitation Facilities), and LTACHs (Long-Term Acute Care Hospitals).
Daily infection risks remain high, with 1 in 31 hospital patients and 1 in 43 nursing home residents acquiring at least one HAI. In 2023, California (8,024 cases), New York (5,491), and Texas (5,177) recorded the highest HAI counts-reflective of their large patient populations.
HAIs continue to be one of the most common complications of hospital care, with over one million cases annually and an estimated 633,300 patients affected at any given time, driving significant morbidity, mortality, and multi-billion-dollar economic burden.
As prevention emerges as a national priority, federal programs from AHRQ (Agency for Healthcare Research and Quality), CDC (Centers for Disease Control and Prevention), and CMS (Centers for Medicare & Medicaid Services) are accelerating adoption of evidence-based antiseptic protocols.
Notably, the AHRQ-supported CUSP (Comprehensive Unit-based Safety Program) model, combining culture improvement, teamwork, and checklists, has achieved substantial reductions in CLABSIs (Central Line-Associated Bloodstream Infections) and strengthened the case for routine antiseptic bathing as hospitals and nursing homes work to curb evolving HAI patterns.
By 2025, Europe is projected to secure 23.8% of the global antiseptic bathing market, supported by strong regulatory emphasis on reducing HAIs, growing outpatient and home-health antiseptic adoption, and strict accreditation standards that embed routine antiseptic bathing into care protocols.
Central to this momentum are the National Standards of Healthcare Cleanliness 2025, which replace the 2021 framework and apply across all healthcare organizations, including ambulance facilities.
These standards mandate transparent cleaning practices, detailed compliance grids, contract-bound auditing, and alignment with modern IPC (Infection Prevention and Control) requirements, as reinforced by Regulation 13 of the Health and Social Care Act 2022. Their focus on flexibility, functional-risk assessment, and pandemic-responsive cleaning has strengthened expectations for consistent, high-quality hygiene.
Clinical evidence further supports market expansion. Octenidine, widely used across Europe, has demonstrated broad-spectrum activity against Gram-positive and Gram-negative bacteria and has no documented resistance or significant side effects.
A Germany-wide EFFECT study evaluating octenidine-based antiseptic bathing in ICUs confirmed its effectiveness in preventing ICU-acquired primary bacteremia, particularly from Gram-positive organisms and skin commensals. When incorporated into bundled infection-prevention strategies, octenidine provides a strong alternative to chlorhexidine and reinforces Europe’s push to reduce nosocomial infections and enhance patient safety.
Demand for antiseptic bathing accessories in the Asia Pacific is expanding and is projected to attain a CAGR of 5.8%. Rapid healthcare infrastructure development across India, China, and Japan is boosting the adoption of antiseptic bathing as hospitals scale preoperative and ICU infection-prevention protocols.
Rising surgical volumes, particularly in oncology, cardiology, orthopedics, and transplant centers, are driving routine use of antiseptic wipes and CHG/octenidine-based bathing to reduce perioperative infection risk.
Regional surgery-focused initiatives-driven by WHO frameworks and expanding national surgical plans-are further strengthening perioperative and critical-care systems across the Asia Pacific, indirectly accelerating demand for antiseptic bathing to standardize infection prevention in rapidly growing surgical and ICU environments.
Additionally, expanding critical-care capacity, including new ICUs and high-dependency units, has increased demand for standardized bathing practices to reduce device-associated infections. Meanwhile, growing local production of antiseptic formulations is also lowering costs, making adoption feasible for both public and private hospitals across emerging markets.
Coupled with the region’s strong position in medical tourism, accounting for 45.7% of global share in 2025, Asia Pacific’s growing surgical demand and investment in essential care services continue to accelerate antiseptic bathing adoption across hospitals and ICUs.
The antiseptic and disinfecting wipes market is increasingly focused on sustainability and eco-friendly innovations, with several new launches emphasizing 100% plant-based or biodegradable materials. Recent products offer reduced plastic usage, compostable substrates, and environmentally safer active ingredients such as citric acid, while maintaining high germ-kill efficacy.
These innovations target hospital and critical-care settings, enabling rapid disinfection with reduced waste. The shift toward plastic-free and recycled packaging reflects rising regulatory and consumer demand for environmentally responsible healthcare products, creating a competitive environment where efficacy, safety, and sustainability are key differentiators in market positioning.
The global antiseptic bathing market is valued at US$ 16.4 Billion in 2025.
Rising hospital-acquired infections (HAIs), expanding ICU and surgical volumes, and stringent infection-control protocols globally drive market growth.
The global market is poised to witness a CAGR of 4.7% between 2025 and 2032.
Adoption of eco-friendly, plant-based, and bundled antiseptic bathing solutions in hospitals and high-risk care settings present major growth opportunities.
Major players in the global are Ecolab Inc., BD, 3M, The Clorox Company, Medline Industries, LP. and others.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author