ID: PMRREP34750| 200 Pages | 10 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

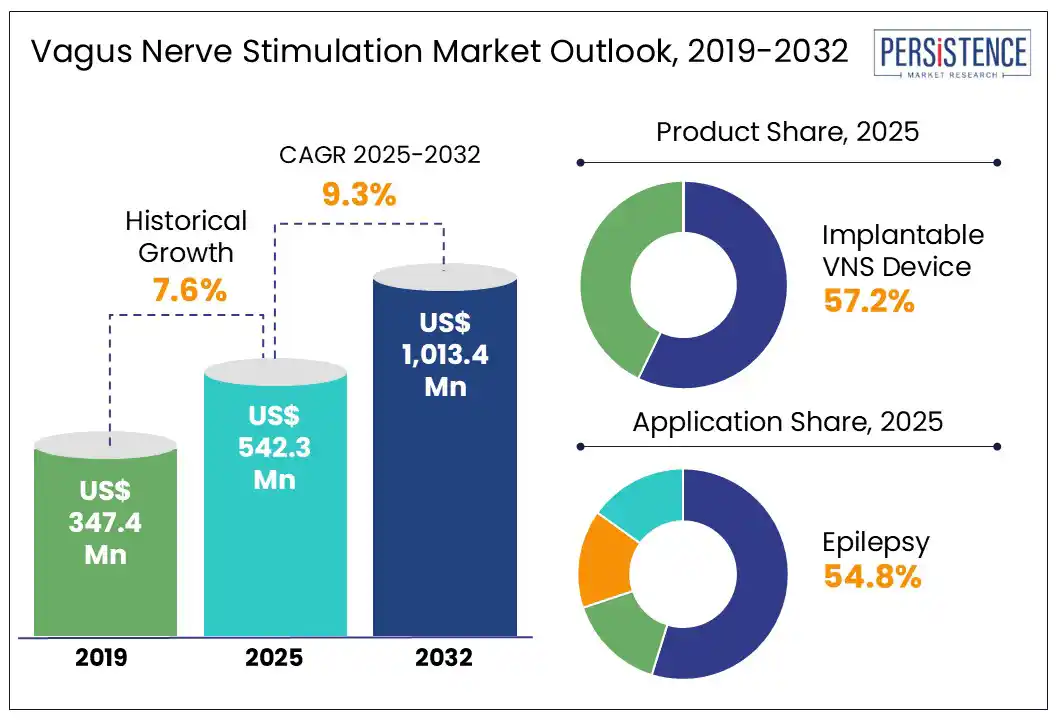
The global vagus nerve stimulation market size is likely to be valued at US$542.3 Mn in 2025 and is expected to reach US$1,013.4 Mn by 2032, growing at a CAGR of 9.3% during the forecast period from 2025 to 2032.
Vagus nerve stimulation market is experiencing significant growth due to the rising prevalence of neurological and other chronic diseases such as epilepsy, depression, and migraines. Vagus nerve stimulation therapy has proven efficacy in cases resistant to treatment and is increasingly adopted due to advancements in device technology and expanding clinical applications. Favourable regulatory frameworks and growing awareness among healthcare providers are also enhancing market penetration. Furthermore, ongoing research and developments of non-invasive and personalized stimulation approaches are opening new therapeutic avenues, positioning the global vagus nerve stimulation for sustained expansion in the coming years.
Key Industry Highlights:
|
Global Market Attribute |
Key Insights |
|
Vagus Nerve Stimulation Market Size (2025E) |
US$ 542.3 Mn |
|
Market Value Forecast (2032F) |
US$ 1,013.4 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
9.3% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.6% |
The global vagus nerve stimulation market is growing steadily with the increasing prevalence of neurological disorders, including epilepsy, depression, and chronic migraines, especially among the geriatric population. Recent studies show that around 65 million individuals are affected by epilepsy globally, with 80% living in developing countries. Nearly 30-40% of epileptic patients suffer from drug-resistant epilepsy (DRE).
Vagus nerve stimulation (VNS) is increasingly recognized as an effective therapy for treatment-resistant epilepsy. According to the International League Against Epilepsy (2025), one-third of patients continue to experience seizures despite medication. Clinical studies have shown VNS to reduce seizure frequency by up to 36% after 6 months, 58% after 4 years, and 75% by 10 years. Additionally, patients reported improved seizure recovery, reduced severity, enhanced alertness, and better quality of life. Such clinical evidence is driving wider adoption of VNS therapy.
The advancements in technology, such as closed-loop systems and AI-enabled personalization, are further improving treatment results and increasing uptake. The scope of application is expanding beyond epilepsy and depression to stroke rehabilitation and chronic heart failure, and more. In May 2024, UConn Health became the first in Connecticut to implant the FDA-approved Vivistim® Paired VNS™ System, improving upper limb function in stroke survivors.
Similarly, in June 2024, electroCore launched TAC-STIM™, a non-invasive VNS device developed with the U.S. military to enhance human performance. These advances, along with expanding therapeutic indications, are fuelling sustained growth across the Vagus nerve stimulation.
A key restraint in the global vagus nerve stimulation (VNS) market is the limited awareness and acceptance among both healthcare providers and patients. Although VNS has shown significant efficacy in managing conditions such as epilepsy and depression, it remains overshadowed by more conventional treatments. This is partly due to insufficient clinical familiarity and perceived complexity in use.
On the patient side, concerns about surgical procedures, potential side effects, and lack of information further reduce uptake. These issues are compounded by gaps in clinical education and limited exposure to neuromodulation therapies during medical training. As a result, VNS is often underutilized, even in cases where it could offer significant benefits. Addressing these barriers through targeted education and training is essential to expand the market and improve patient outcomes.
Expanding into emerging markets offers a significant growth opportunity for the vagus nerve stimulator device manufacturers. Significant improvements in healthcare infrastructure across Asia Pacific, Latin America, and Africa, coupled with rising neurological disorder prevalence, highlight a critical unmet need. According to World Health Organization (WHO) 2024 statistics, globally one in three individual is affected by neurological conditions and more than 80% of neurological deaths and health loss occur in low- and middle-income countries (LMICs).
Introducing VNS therapy in these regions are anticipated to address care gaps, especially when supported by collaborations with local healthcare systems and NGOs. Cost reduction through local manufacturing and distribution partnerships are likely to further enhance accessibility and affordability.
Additionally, in 2024, Teliatry introduced the ReStore batteryless, wireless neurostimulator, developed with the Texas Biomedical Device Center (TXBDC), receiving FDA approval for pilot studies targeting Post-traumatic stress disorder (PTSD) and spinal cord injury recovery. Such innovations, combined with telemedicine integration, present scalable, low-barrier VNS solutions tailored to remote or underserved population.
Implantable VNS devices are projected to capture a dominant revenue share of around 57.2% in 2025, driven by their established clinical efficacy, long-term therapeutic outcomes, and widespread use in managing treatment-resistant epilepsy and depression. These devices are supported by strong clinical data and are often preferred in severe or chronic cases where continuous stimulation is required. However, the external non-invasive VNS device segment is the fastest-growing, fuelled by increasing demand for non-surgical treatment options and the expanding application of these devices in conditions such as migraine and depression, offering a more accessible alternative to invasive procedures.
Epilepsy segment is expected to hold a significant revenue share in the global vagus nerve stimulation market in 2025. This is largely due to the long-standing clinical use and regulatory approval of VNS therapy as an adjunctive treatment for drug-resistant epilepsy. Compared to other indications, such as depression, migraine, or inflammatory disorders, epilepsy has the most established evidence base and patient population for VNS devices. While applications in depression and migraine are growing rapidly, especially with non-invasive devices, epilepsy remains the primary therapeutic area where VNS is routinely integrated into standard care protocols.
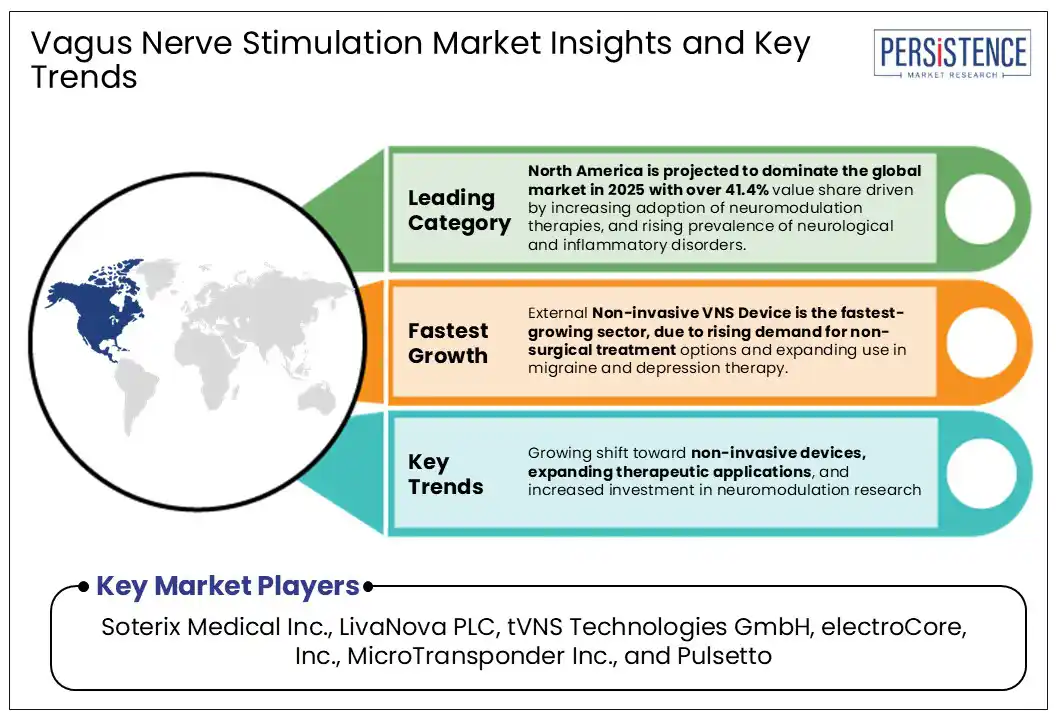
North America is anticipated to dominate the global market in 2025 with 41.4% value share, driven by increasing adoption of neuromodulation therapies and high prevalence of neurological and inflammatory disorders. The region benefits by accelerating the early adoption of VNS devices. Favourable reimbursement policies for epilepsy and depression improve accessibility, while high healthcare expenditure supports the development of advanced neuromodulation treatments.
Rigorous regulatory standards and strong clinical evidence expedite approval and market entry of novel VNS systems. Recent clinical advancements include the February 2024 VNS-REHAB pivotal trial at the American Stroke Association’s International Stroke Conference 2024, which revealed that combining VNS with intense physical rehabilitation significantly improved arm and hand function in stroke survivors.
Major clinical trials have demonstrated VNS effectiveness in relieving severe depression, potentially expanding insurance coverage for treatment-resistant cases. In May 2024, Tivic Health® announced the successful completion of a non-invasive cervical VNS study with The Feinstein Institutes, confirming its potential across autonomic, cardiac, and central nervous system disorders, further reinforcing North America’s leadership in the vagus nerve stimulation market.
Europe is rapidly emerging as a key market for VNS, driven by innovative non-invasive technologies and growing clinical adoption. Germany's vagus nerve stimulation market is at the forefront through a collaboration such as tVNS® and Pansatori. The alliance offers an integrated system combining the ForgTin® ear clamps, Tilecio app, and tVNS® E device, all clinically validated and approved for tinnitus treatment. The tVNS® device is notable for stimulating 100% of the vagus nerve via the cymba concha and is the only non-invasive VNS device approved as a Class IIa medical device under the EU-MDR (European Union Medical Device Regulation).
Meanwhile, the UK vagus nerve stimulation market is advancing through personalized VNS therapies, exemplified by the 2024 TinnSpire study exploring combined vagus nerve and sound stimulation to treat tinnitus. These innovations, backed by strong regulatory approvals and patient-centric digital tools, are driving awareness and expanding the vagus nerve stimulation across Europe.
Asia Pacific market is rapidly growing, driven by innovative developments and rising healthcare needs. In Japan, companies such as Adriakaim are pioneering VNS technology aimed at preventing chronic heart failure, a serious concern affecting approximately 23,000 patients annually after an acute myocardial infarction. Their device uses a unique, low-intensity electrical stimulus to target specific organs, offering a novel treatment approach beyond conventional drugs. This “people-friendly” technology promises to extend healthy life expectancy while reducing medical costs.
Meanwhile, in Australia, the Victorian Government’s US$500,000 grant awarded in June 2024 to the Bionics Institute is accelerating clinical trials for a VNS device designed to modulate immune responses and prevent long-term damage. These advancements, combined with increasing awareness and investment in medical technology, addressing unmet clinical needs, and fostering cutting-edge therapeutic innovations across the region, have propelled the industry expansion.
The global vagus nerve stimulation market is rapidly evolving with advanced device launches, increased funding, and dedicated centers of excellence. Growing focus on stroke recovery, neurological disorders, and clinical education is driving innovation and expanding access to vagus nerve stimulation therapies globally.
The global vagus nerve stimulation market is set to reach US$ 542.3 Mn in 2025.
The market is projected to record a CAGR of 9.3% during the forecast period from 2025 to 2032.
Growing shift toward non-invasive devices, expanding therapeutic applications, and increased investment in neuromodulation research are driving the global market.
Soterix Medical Inc., LivaNova PLC, tVNS Technologies GmbH, electroCore, Inc., MicroTransponder Inc., and Pulsetto are a few leading players.
North America is projected to dominate the global market in 2025.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Billion |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author