ID: PMRREP34631| 195 Pages | 10 Feb 2026 | Format: PDF, Excel, PPT* | Healthcare

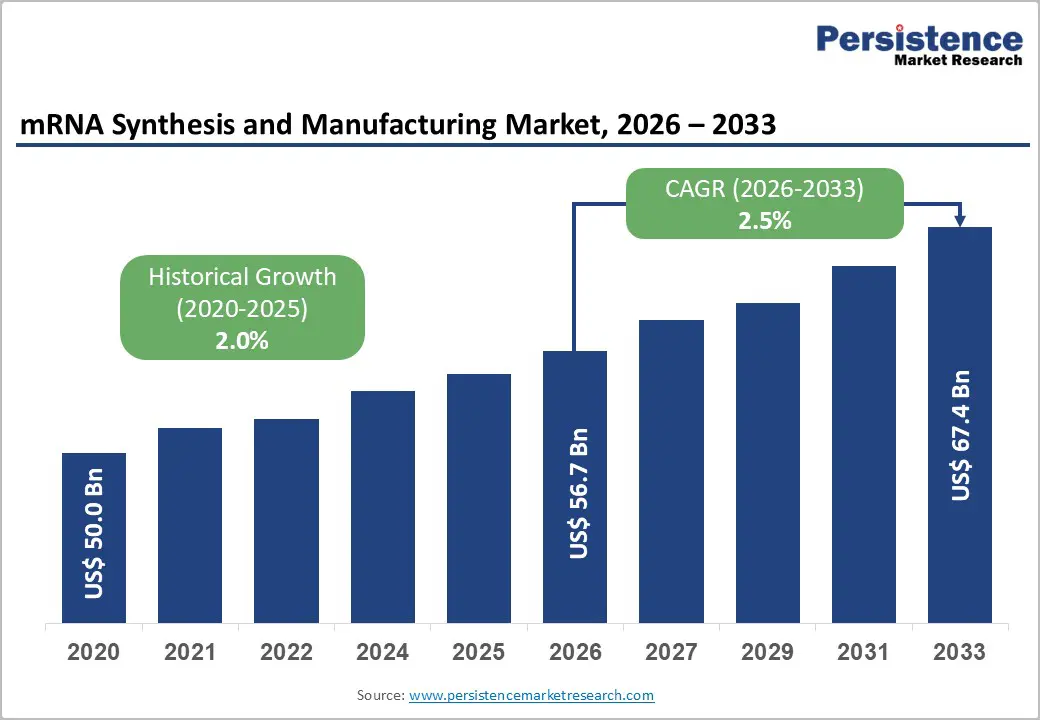
The global mRNA synthesis and manufacturing market size is likely to be valued at US$ 56.7 billion in 2026 to US$ 67.4 billion by 2033 growing at a CAGR of 2.5% during the forecast period from 2026 to 2033.
The global market is growing steadily, driven by the rising adoption of advanced therapeutics, automation, and data-driven manufacturing. North America leads owing to robust biopharmaceutical infrastructure and regulatory rigor. Asia-Pacific is the fastest-growing region, supported by expanding manufacturing capacity, government support, a skilled workforce, and increasing investments in scalable RNA production technologies.
| Key Insight | Details |
|---|---|
| mRNA Synthesis and Manufacturing Market Size (2026E) | US$ 56.7 Bn |
| Market Value Forecast (2033F) | US$ 67.4 Bn |
| Projected Growth (CAGR 2026 to 2033) | 2.5% |
| Historical Market Growth (CAGR 2020 to 2025) | 2.0% |
The rapid advancements in mRNA technology are revolutionizing the biopharmaceutical industry. Innovations in mRNA synthesis and manufacturing, particularly the development of sophisticated lipid nanoparticle delivery systems, are pivotal in driving market growth.
Such advancements enhance the stability, efficacy, and delivery efficiency of mRNA-based therapeutics and vaccines. The ability to precisely engineer mRNA sequences and optimize delivery mechanisms has significantly expanded the therapeutic potential of mRNA technology beyond vaccines to include treatments for various diseases, such as cancer, genetic disorders, and autoimmune conditions. Continuous innovation improves patient outcomes and attracts substantial investment and collaboration across industry sectors, fostering a robust ecosystem for further development and commercialization.
The complexity of manufacturing steps poses a significant restraint for mRNA synthesis and manufacturing. The process involves intricate steps, including mRNA synthesis, purification, and formulation into lipid nanoparticles, each requiring precise control and specialized equipment.
Ensuring consistency and scalability across production batches adds further challenges. Moreover, stringent regulatory requirements for quality control and validation increase operational complexities and costs. These factors collectively limit the speed and scale of production, potentially delaying time-to-market for new therapies. Addressing these challenges requires sustained investment in automation, process optimization, and regulatory compliance to streamline manufacturing and effectively meet growing global demand.
Regulatory support and accelerated approvals are crucial for advancing the market for mRNA synthesis and manufacturing. Close collaboration between industry pioneers and regulatory bodies is essential to establish robust guidelines and expedite approval processes for mRNA-based therapies.
The proactive engagement not only accelerates the time to market for new treatments but also ensures that stringent safety and efficacy standards are met. Furthermore, funding initiatives undertaken by regulatory bodies play a pivotal role in accelerating the development and production of mRNA technologies. Such an initiative not only supports advances in vaccine and therapeutic development but also reinforces the FDA's commitment to fostering a robust ecosystem for mRNA technologies.
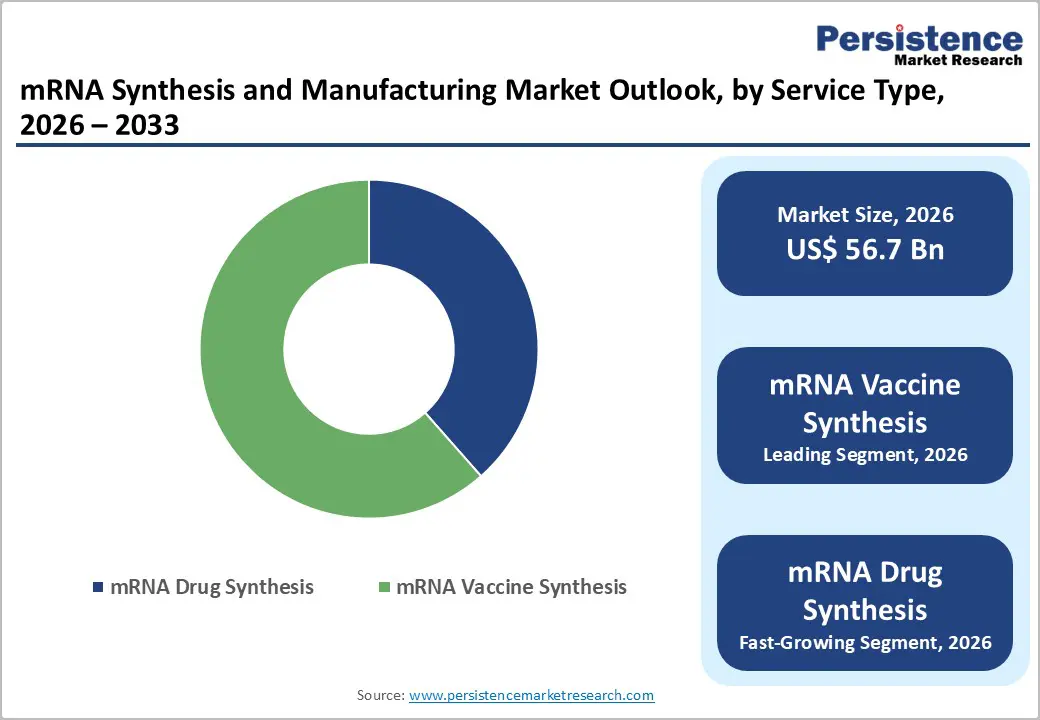
mRNA vaccine synthesis occupies 61.5% share of the global market in 2025, because vaccines represent the highest-volume application for RNA production, driving sustained demand for large-scale synthesis services. Real-world immunization data show that more than 11 billion COVID-19 vaccine doses of all types were administered globally by early 2022, with mRNA platforms accounting for roughly 43 % of production volume during peak pandemic manufacturing efforts, reflecting unparalleled scale relative to other biologics. This massive deployment established mRNA vaccine workflows, infrastructure, and regulatory pathways, creating a dominant service demand that translates into the largest share of mRNA synthesis activity worldwide.
By scale of operation, commercial production dominates the mRNA synthesis and manufacturing market because industrial-level output far exceeds preclinical and clinical needs, anchored by worldwide demand for licensed mRNA vaccines and therapeutics. Government and industry data indicate that commercial-scale operations account for more than 85% of total mRNA synthesis and manufacturing activity, reflecting the prioritization of large-volume vaccine and therapeutic supply chains over smaller research batches. This predominance arises because commercial production requires rigorous GMP compliance, high-throughput facilities, and consistent quality to meet regulatory approval and global immunization programs, capacities that research or clinical-scale labs cannot match. The volume of commercial mRNA vaccine manufacture, exemplified by billion-dose production capacities at companies such as Pfizer/BioNTech and Moderna, underscores the scale imbalance between commercial and early-stage synthesis.
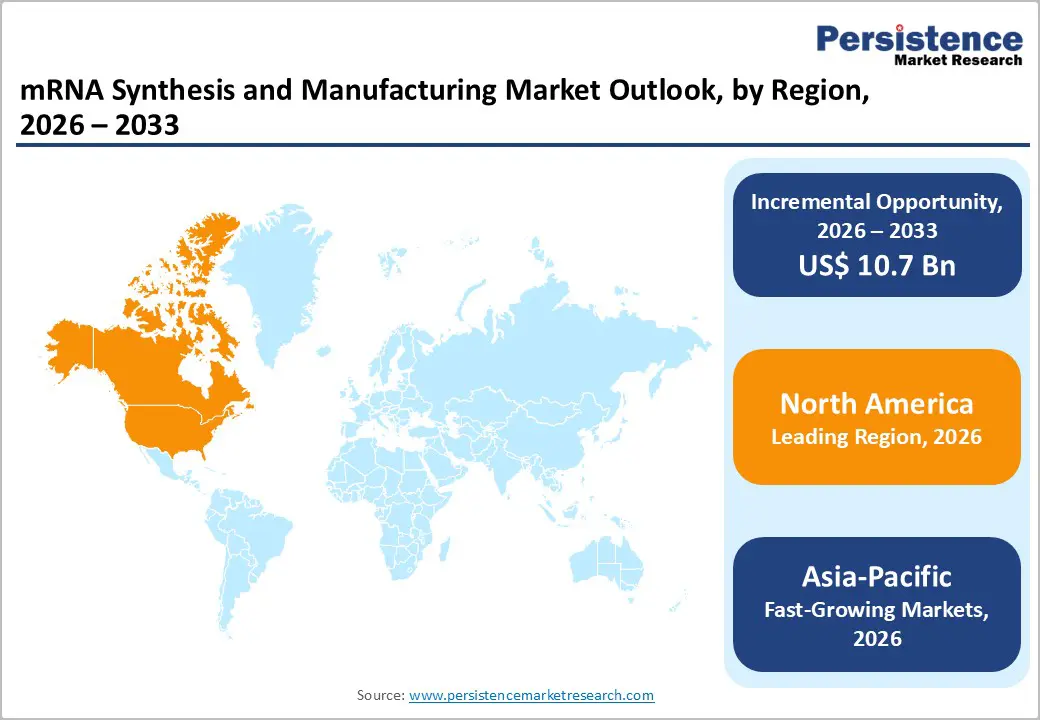
North America dominates the mRNA synthesis and manufacturing market with 42.6% share in 2025, because its biopharmaceutical and vaccine production infrastructure is significantly more developed than in other regions, supported by concentrated industry hubs, public funding, and regulatory systems that enable rapid development and scale-up. U.S. government investment and pandemic response programs have historically mobilized tens of billions of dollars to develop, manufacture, and purchase mRNA vaccines, with about 92 % of fully vaccinated Americans receiving an mRNA-based COVID-19 vaccine, indicating the scale of domestic mRNA deployment. Additionally, the United States contributes the majority of North American vaccine production capacity, with around 80 % of North America’s vaccine contract manufacturing centered in U.S. hubs like Massachusetts, California, and North Carolina, reflecting robust domestic synthesis and manufacturing capabilities.
Europe is an important region in the mRNA synthesis and manufacturing market because it combines strong biotechnology infrastructure, coordinated regulatory frameworks, and significant public and private investment in RNA technologies. The European Medicines Agency (EMA) has played a central role in authorizing multiple mRNA vaccines, demonstrating the region’s capacity to evaluate complex RNA products efficiently. European countries such as Germany and Belgium house major mRNA production facilities, including those used for pandemic vaccine manufacture, and have established networks of biotech clusters and CDMOs that support both early-stage synthesis and large-scale manufacturing. Additionally, European Union initiatives on health security and pandemic preparedness have prioritized domestic RNA production capabilities, reinforcing the region’s strategic importance in global mRNA manufacturing.
Asia Pacific is the fastest-growing region in the mRNA synthesis and manufacturing market because governments and manufacturers are rapidly expanding local capabilities to meet both domestic and global demand. Major biopharma firms such as BioNTech have established first-of-their-kind GMP mRNA manufacturing facilities in Singapore, expected to produce up to several hundred million doses annually and create over 100 jobs, illustrating expanded regional capacity. Surveys of regional vaccine manufacturers show that 87 % view mRNA technology as a key future modality, with more than 60 % planning new or expanded mRNA facilities over the next few years, reflecting strong industry commitment to growth. Moreover, countries such as China are increasing clinical activity with dozens of approved mRNA-related trials, reinforcing sustained expansion of synthesis and manufacturing infrastructure.
Leading mRNA synthesis and manufacturing companies prioritize scalable IVT production, advanced formulation, and GMP compliance. Investments in process optimization, AI-driven design, and high-throughput screening enhance yield, stability, and reproducibility. Collaborations with biotech, academia, and regulators accelerate mRNA and siRNA development, while integrated quality control and supply chains support vaccines, therapeutics, and precision medicine globally.
The global mRNA synthesis and manufacturing market is projected to be valued at US$ 56.7 Bn in 2026.
Rising demand for mRNA vaccines, gene therapies, advanced production technologies, and global biopharmaceutical investments drive growth.
The global mRNA synthesis and manufacturing market is poised to witness a CAGR of 2.5% between 2026 and 2033.
Next-generation mRNA, saRNA, siRNA therapeutics, personalized vaccines, scalable platforms, AI optimization, and emerging biotech expansion.
DH Life Sciences, LLC (Danaher), Azenta Life Sciences (Genewiz), Merck KGaA, TriLink BioTechnologies, GenScript, Creative Biolabs.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Service Type
By Scale of Operation
By Therapeutic Area
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author