ID: PMRREP22768| 199 Pages | 4 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

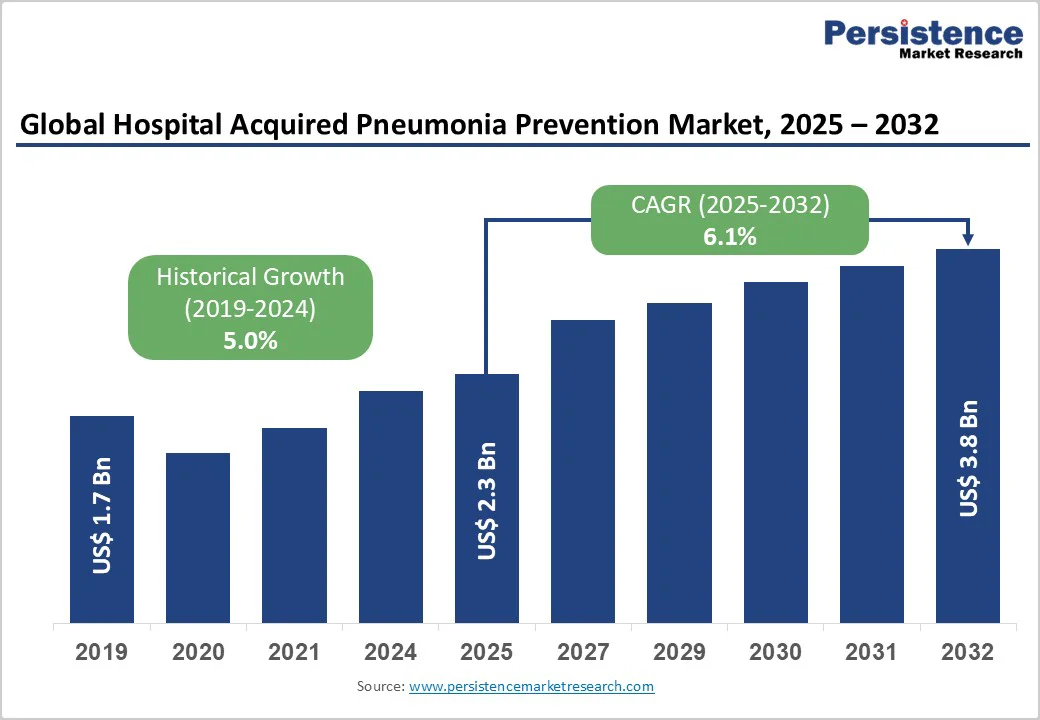
The global hospital acquired pneumonia prevention market size is valued at US$ 2.3 billion in 2025 to US$ 3.8 Billion by 2032. The market is projected to record a CAGR of 6.1% during the forecast period from 2025 to 2032.
Global demand for hospital-acquired pneumonia prevention solutions is rising as healthcare facilities increasingly adopt standardized infection-control protocols, advanced oral-care kits, and antimicrobial hygiene devices. Growing awareness of non-ventilator hospital-acquired pneumonia (HAP), stricter regulatory emphasis on patient safety, and the integration of disposable, contamination-free oral-care products are accelerating market expansion.
Additionally, improvements in hospital infrastructure, rising ICU admissions, and the prioritization of evidence-based infection-prevention programs across developed and emerging economies are increasing adoption of products and services for HAP-prevention.
| Global Market Attributes | Key Insights |
|---|---|
| Hospital Acquired Pneumonia Prevention Market Size (2025E) | US$ 2.3 Billion |
| Market Value Forecast (2032F) | US$ 3.8 Billion |
| Projected Growth (CAGR 2025 to 2032) | 6.1% |
| Historical Market Growth (CAGR 2019 to 2024) | 5.0% |
The increasing rate of ICU admissions, prolonged ventilation support, and extended hospital stays is significantly elevating the global incidence of hospital-acquired pneumonia (HAP). This trend is pushing hospitals to implement standardized oral-care bundles, antimicrobial hygiene tools, and single-use prevention kits to minimize complications and mortality.
Strengthened awareness efforts by the Centers for Disease Control and Prevention (CDC), National Health Service (NHS), and Ministry of Health and Family Welfare (MoHFW) are further improving institutional commitment to infection control, encouraging higher spending on HAP-prevention technologies across acute and critical-care units.
For instance, the 2023 HAP Policy Project reported 412,048 HAP cases in England between April 2018 and March 2023 with an annual average of 82,410 cases.
Hospitals are increasingly moving away from conventional, manually assembled oral-care practices toward fully sealed, disposable, and pre-packaged oral-care kits. These kits typically containing suction toothbrushes, foam swabs, moisturizers, antiseptic rinses, and single-use hygiene components help reduce cross contamination and ensure consistent care protocols across nursing teams.
The rising emphasis on infection prevention, compliance with hospital quality standards, and the need to streamline oral-care workflows in ICUs and high-risk wards are accelerating the adoption of these ready-to-use kits, thereby driving market growth.
Oral-care protocols for preventing hospital-acquired pneumonia continue to face major implementation challenges across clinical settings. High nursing workloads, staffing shortages, and inconsistent hands-on training often lead to poor compliance with recommended oral-care frequencies and procedures.
In many hospitals, oral hygiene is deprioritized compared with other urgent interventions, resulting in variability in care delivery and reduced overall effectiveness of HAP-prevention programs.
Moreover, growing clinical concerns regarding chlorhexidine based oral solutions including mucosal irritation, hypersensitivity reactions, and uncertainty surrounding long-term safety are prompting hospitals to reassess product usage.
Some healthcare facilities are shifting toward alternative formulations such as sodium bicarbonate or non-medicated moisturizers to avoid adverse effects. Together, these workforce limitations and product-safety concerns are slowing the adoption of advanced oral-care bundles and limiting broader market penetration.
The rising demand for gentle, non-irritating oral-care formulations is creating significant opportunities for manufacturers. Hospitals and elder-care facilities are increasingly shifting away from chlorhexidine-based and alcohol-containing products due to concerns over mucosal irritation, hypersensitivity, and patient discomfort.
This shift is fueling innovation in aloe-vera gels, pH-balanced rinses, herbal antimicrobial blends, and fluoride-free cleansers tailored for fragile, intubated, or post-operative patients. Companies that invest in clinically validated chlorhexidine gluconate (CHG)-free portfolios stand to capture growing preference for safer, patient-friendly alternatives.
Furthermore, the rising geriatric population and increasing rates of chronic illness are driving higher demand for oral-care solutions in long-term care homes, nursing facilities, and home-healthcare services. Bedridden and dependent patients face an elevated risk of aspiration and hospital-acquired pneumonia, creating strong market potential for disposable oral kits, moisturizer gels, suction toothbrushes, and portable suction systems.
As governments strengthen policies for elderly care and home-based management of chronic conditions, suppliers offering easy-to-use, single-use, caregiver-friendly oral-care bundles are positioned for rapid adoption across these expanding care environments.
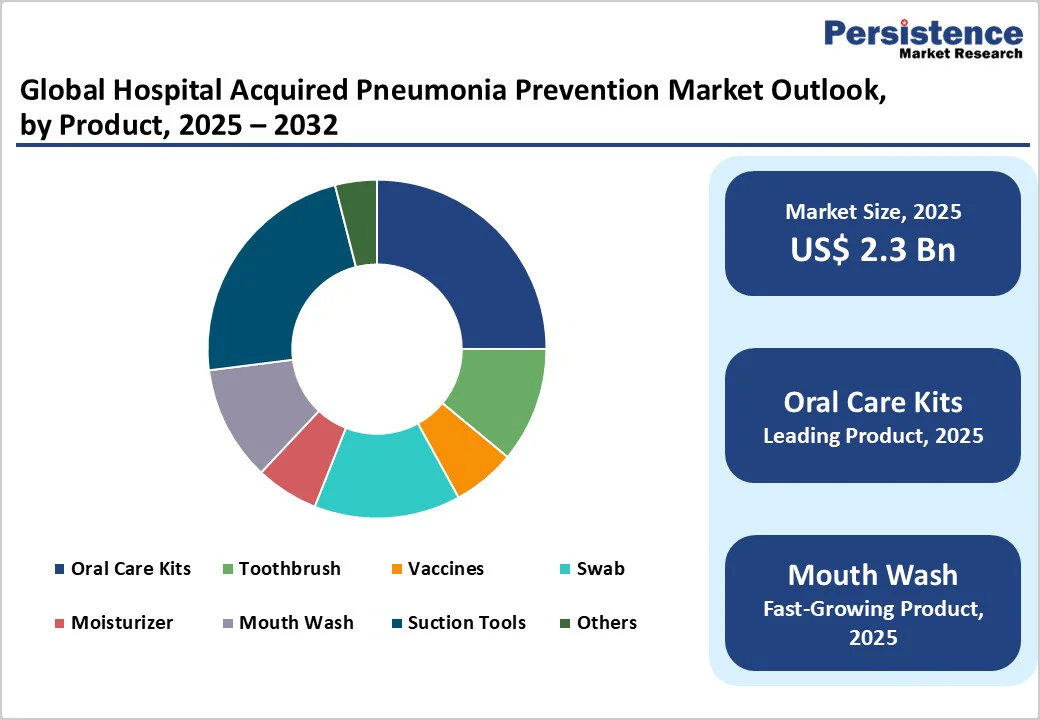
The oral care kit segment is projected to dominate the global hospital acquired pneumonia prevention market in 2025, accounting for a revenue share of 25.0%.
The segment’s strong performance is primarily driven by comprehensive composition, convenience, and ability to standardize oral hygiene protocols in ICU and non-ICU settings. These pre-assembled kits include suction toothbrushes, swabs, moisturizers, and antiseptic mouthwashes, ensuring complete and consistent care while reducing variability in nursing practices.
Hospitals prefer them because they minimize contamination risks, improve protocol compliance, and significantly lower the likelihood of micro-aspiration and pneumonia in ventilated patients. Their ease of use, time efficiency, and strong alignment with CDC and World Health Organization (WHO) oral-care recommendations
The hospitals segment is projected to dominate the global hospital acquired pneumonia prevention market in 2025, accounting for a revenue share of 56.6%. This is due to the high volume of critically ill and ventilated patients, strict compliance requirements for infection-prevention protocols, and widespread adoption of standardized oral-care bundles to reduce HAP and ventilator-associated pneumonia incidence.
Large multispecialty hospital networks allocate significant budgets for ICU hygiene products, including oral care kits, suction tools, and antiseptic solutions, ensuring consistent demand. Additionally, frequent audits by regulatory bodies and the need to reduce the length of stay, mortality risk, and treatment costs.
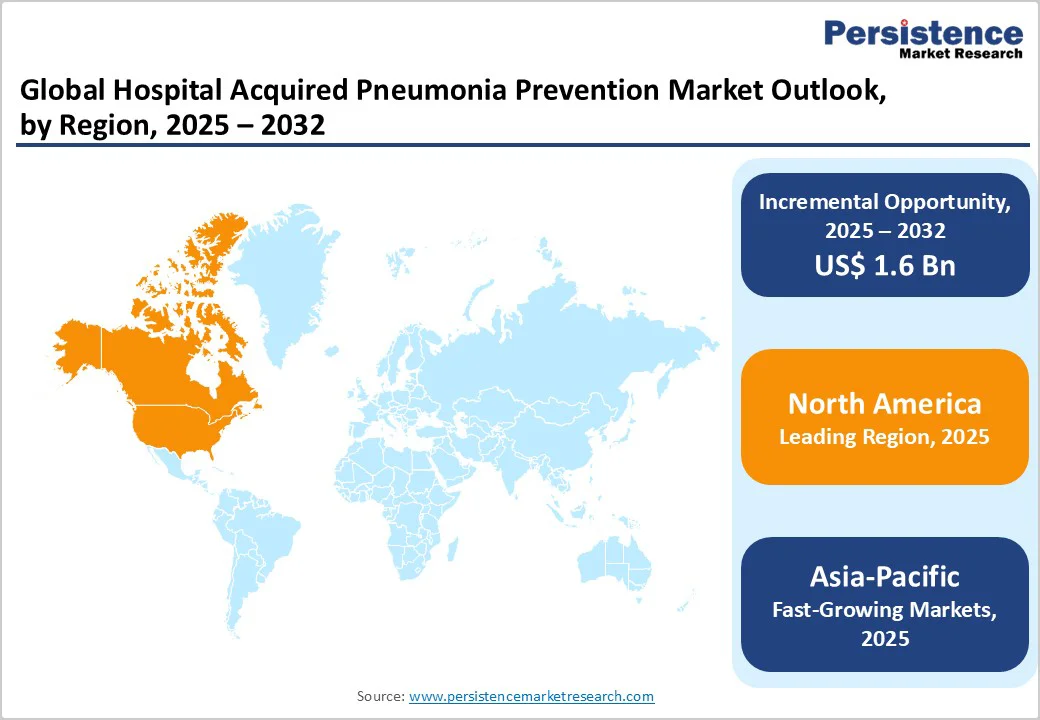
The North America market is expected to dominate globally with a value share of 38.4% in the 2025, with the U.S. leading the region due to its highly developed hospital infrastructure, strong ICU admission volumes, and strict infection-prevention regulations enforced by CDC and Joint Commission.
Growing adoption of standardized oral-care bundles, expanding use of disposable suction toothbrushes and CHG-based antiseptic gels, and rising focus on preventing ventilator-associated pneumonia in critical-care settings are further strengthening market growth.
Additionally, high healthcare spending, rapid integration of AI-enabled monitoring tools, and presence of major manufacturers position the region as the most mature and innovation-driven market for HAP prevention solutions.
The Europe market is projected to hold 27.6% in 2025, and is expected to grow steadily, driven by strict infection-prevention regulations enforced by the European Centre for Disease Prevention and Control (ECDC) and national health agencies, along with increasing adoption of standardized oral-care protocols across ICUs and long-term care facilities.
Rising geriatric hospitalizations, higher prevalence of ventilator-associated complications, and growing preference for CHG-free and alcohol-free oral hygiene solutions are contributing to regional demand.
Additionally, strong government-backed healthcare-associated infection reduction programs, continuous staff training initiatives, and procurement of pre-packaged disposable oral-care kits are further strengthening Europe’s position as a progressive, compliance-focused market for hospital-acquired pneumonia prevention.
The Asia Pacific market is expected to register a relatively higher CAGR of around 8.4% between 2025 and 2032, driven by the rapid expansion of hospital and ICU infrastructure across India, China, and Southeast Asia, along with the rising awareness of infection prevention and control (IPC) standards in public and private hospitals.
Increasing incidence of ventilator-associated pneumonia, growing geriatric and immunocompromised patient populations, and higher adoption of cost-effective disposable oral-care kits are boosting product uptake. Moreover, government-led HAI reduction programs, improving nurse-training practices, and rising procurement of standardized oral-care bundles are strengthening market growth across the region.
The global hospital-acquired pneumonia prevention market is highly competitive, with major players such as Stryker, Medline Industries, LP, Intersurgical Ltd., JASE Medical, and Teleflex Incorporated leading the industry through broad product portfolios, strong worldwide distribution networks, and continuous innovation in oral-care kits, suction systems, antiseptic solutions, and ICU hygiene accessories.
These companies focus on developing advanced HAP-prevention solutions including pre-packaged oral-care bundles, CHG-based antiseptics, suction toothbrushes, and airway management devices to strengthen clinical outcomes and reduce hospital infection rates.
The global hospital acquired pneumonia prevention market is projected to be valued at US$ 2.3 Billion in 2025.
Rising ICU admissions, increased HAP incidence, and the growing adoption of standardized oral-care protocols are driving the global hospital acquired pneumonia prevention market.
The global hospital acquired pneumonia prevention market is poised to witness a CAGR of 6.1% between 2025 and 2032.
Rising demand for CHG-free, alcohol-free oral-care formulations and the rapid expansion of prevention solutions in long-term care and home-healthcare settings are creating significant growth opportunities in the hospital acquired pneumonia prevention market.
Stryker, Medline Industries, LP, Intersurgical Ltd., JASE Medical, and Teleflex Incorporated are some of the key players in the hospital acquired pneumonia prevention market.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author