ID: PMRREP22915| 210 Pages | 30 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

The global defibrillator pads market size is likely to be valued at US$1.4 billion in 2026 and is expected to reach US$2.6 billion by 2033, growing at a CAGR of 8.8% during the forecast period from 2026 to 2033, driven by the rising prevalence of cardiovascular diseases (CVDs), which account for nearly 17.9 million deaths annually, according to the World Health Organization (WHO).
Increasing deployment of automated external defibrillators (AEDs) in public spaces such as airports, schools, sports arenas, and corporate offices is boosting the demand for replacement pads. Hospitals and emergency care centers are also expanding their inventory of defibrillator pads to ensure a timely response to sudden cardiac arrests.
| Key Insights | Details |
|---|---|
|
Defibrillator Pads Market Size (2026E) |
US$1.4 Bn |
|
Market Value Forecast (2033F) |
US$2.6 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
8.8 % |
|
Historical Market Growth (CAGR 2020 to 2025) |
8.6% |
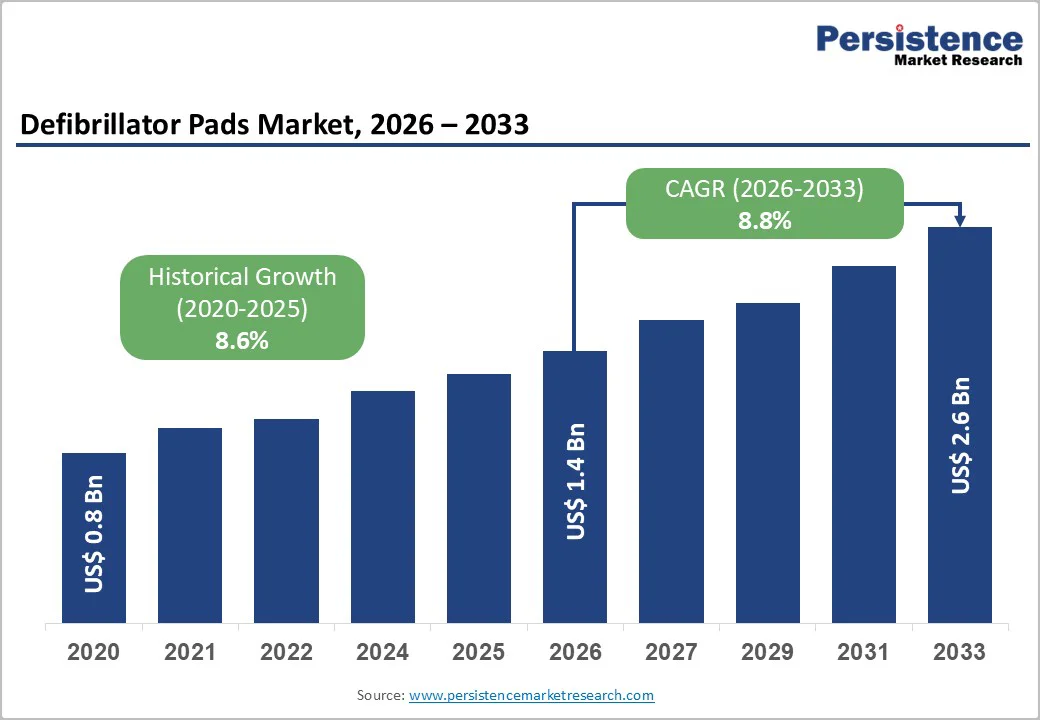
According to the World Health Organization (WHO), CVDs account for nearly 17.9 million deaths annually, representing a leading cause of mortality worldwide. Conditions such as sudden cardiac arrest, heart attacks, and arrhythmias require immediate intervention, often involving defibrillation. This increasing incidence of cardiac events has directly boosted the demand for automated external defibrillators (AEDs) and their consumables, particularly defibrillator pads. Hospitals, emergency medical services, and public access programs are expanding their inventories to ensure timely responses, creating a sustained market for both adult and pediatric defibrillator pads.
The demographic shift toward aging populations globally exacerbates the burden of heart disease as older adults are more susceptible to cardiac events. Technological advancements in pad design, such as hydrogel formulations, self-adhesive applications, and multi-functional compatibility for both adults and children, have reinforced adoption rates. Public awareness campaigns, government initiatives for AED deployment, and regulatory support are complementing market growth, ensuring that emergency defibrillation becomes more accessible.
The production of defibrillator pads relies on a combination of specialized materials such as hydrogel adhesives, conductive substrates, and integrated sensors, often sourced from a limited network of suppliers. Disruptions due to geopolitical tensions, raw material shortages, transportation delays, or pandemic-related bottlenecks can hinder timely manufacturing and delivery. For hospitals, emergency services, and public access programs that depend on consistent stock levels of replacement pads, such interruptions translate into elevated inventory costs, deferred procurement cycles, and challenges in meeting urgent clinical demand.
Regulatory stringency across major markets such as North America, Europe, and parts of Asia adds complexity to product approval and commercialization. Defibrillator pads are classified as medical devices subject to rigorous evaluation by authorities such as the U.S. FDA, EU MDR, and other national bodies to ensure safety, biocompatibility, and performance consistency. Compliance with evolving standards, including clinical validation, labeling requirements, and post-market surveillance, increases development timelines and costs, particularly for new entrants and smaller manufacturers.
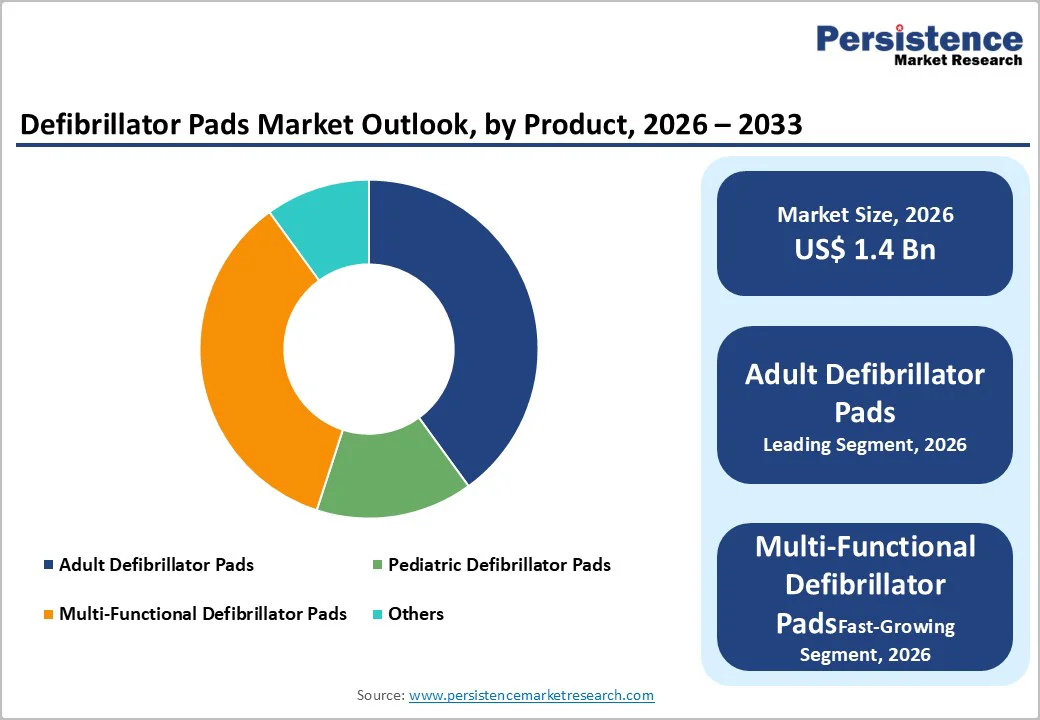
Adult defibrillator pads currently dominate the market, but the rising awareness of sudden cardiac arrest incidents among children has highlighted the need for age-appropriate solutions. Pediatric pads are specifically designed to deliver lower energy levels suitable for children and infants, ensuring safe and effective defibrillation. Hospitals, emergency medical services, and public access programs are increasingly investing in these specialized pads to address the needs of vulnerable populations. This trend is supported by epidemiological data showing that early intervention with pediatric-appropriate pads can dramatically improve survival rates, driving demand for dedicated products.
Technological innovations are enabling the development of multi-functional and specialized pads that cater to both adult and pediatric patients while enhancing ease of use and reliability. Features such as universal compatibility, improved adhesion, and real-time monitoring capabilities allow emergency responders to streamline inventory and reduce response times. Regulatory encouragement for life-saving interventions and growing parental awareness further reinforce the adoption of these pads. Pediatric and specialized pad development represents a promising avenue for manufacturers to differentiate products.
Adult defibrillator pads are expected to lead the defibrillator pads market, accounting for approximately 80% of total revenue in 2026, driven by the higher incidence of cardiac arrests among adult populations. Epidemiological studies indicate that adults account for the majority of out-of-hospital cardiac events, making adult-specific pads essential in both clinical and public access AED programs. Hospitals and emergency services prioritize stocking adult pads to ensure immediate and effective defibrillation during critical events. For example, Philips Healthcare’s HeartStart adult defibrillator pads are widely adopted in U.S. hospitals and public AED installations, reflecting market preference for adult-focused solutions that ensure high efficacy and reliability.
Multi-functional defibrillator pads are likely to represent the fastest-growing segment in 2026, due to their ability to serve both adult and pediatric patients with a single electrode set, simplifying inventory and enhancing emergency response efficiency. For example, Multifunction Defibrillator/AED Pads (One Pair), which offers universal compatibility across age groups and reduces the need for separate adult and pediatric pad stocks. Increasing deployment of public-access AEDs and the growing emphasis on rapid defibrillation in emergency protocols further drive adoption.
Self-adhesive pads are projected to lead the market, capturing around 60% of the total revenue share in 2026, driven by their ease of application and reliability in high-pressure emergency scenarios. These pads are widely used in AEDs across hospitals, clinics, and public spaces, enabling quick attachment to patients without additional preparation. For example, Cardiac Science’s Powerheart AED pads utilize self-adhesive technology that ensures consistent skin contact and reduces application errors during sudden cardiac arrests. Their popularity is reinforced by the growing deployment of public-access AED programs and increased training of first responders, which emphasizes pads that can be rapidly and effectively applied under stress.
Pre-connected pads are likely to be the fastest-growing pad configuration in 2026, due to their ability to reduce response times during critical situations. By being permanently attached to AED leads, they eliminate the need for manual connection, enabling immediate defibrillation. For example, Stryker’s Lifepak pre-connected pads allow emergency personnel to start treatment almost instantly, aligning with regulatory emphasis on rapid defibrillation. Adoption is accelerated by integration with modern AED models and increasing investment in emergency preparedness programs. The efficiency benefits and time-saving advantages in life-threatening situations make pre-connected pads highly attractive for EMS providers and hospitals.
Hydrogel pads are projected to lead the market, capturing around 60% of the total revenue share in 2026, driven by their superior adhesion and consistent conductivity across diverse skin types. Their design minimizes irritation while ensuring effective energy delivery during defibrillation, making them the preferred choice in clinical and public settings. For instance, Medtronic’s Lifepak Hydrogel Pads are widely used in hospitals and emergency settings, offering reliable performance and patient comfort. The growing adoption of AEDs drives the preference for hydrogel pads, the expansion of emergency response infrastructure, and the need for products that perform consistently under varied environmental conditions.
Dry electrodes are likely to be the fastest-growing material composition in 2026, due to their longer shelf life, ease of storage, and suitability for remote or home settings. They eliminate the need for wet gels while maintaining effective conductivity, making them ideal for portable AEDs and consumer-use devices. For example, 3M’s dry electrode pads are gaining popularity in home healthcare markets and remote clinics. Advancements in dry conductive materials, combined with the rising trend of at-home cardiac monitoring and first-aid preparedness, are driving adoption. As healthcare systems encourage wider AED accessibility and convenience, dry electrodes are projected to achieve higher growth rates compared to hydrogel pads.
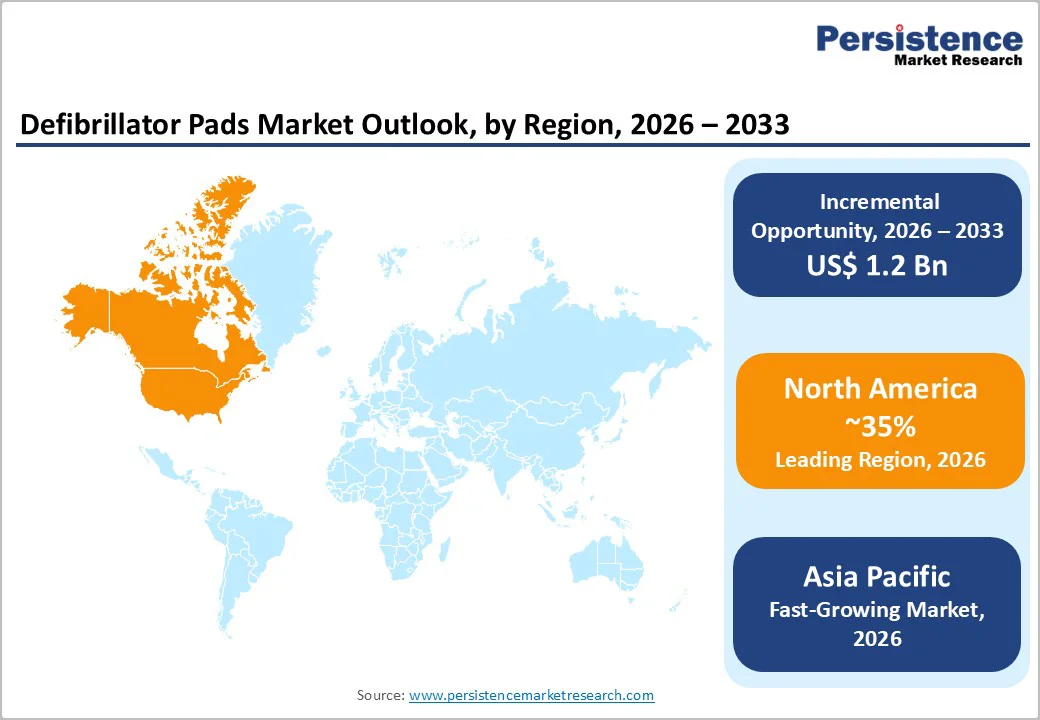
North America is anticipated to be the leading region, accounting for a market share of 35% in 2026, supported by widespread adoption of automated external defibrillators (AEDs), a dense emergency medical services network, and a strong healthcare infrastructure. The U.S. remains the dominant contributor, with hospitals, clinics, EMS providers, and public access programs driving consistent pad replacement cycles and recurrent demand. For example, Philips HeartStart AED Defibrillator Adult SMART Pads Cartridge are widely used in North American hospitals and public-access AED programs, providing reliable, easy-to-apply hydrogel pads compatible with Philips HeartStart AED models and supporting rapid response in sudden cardiac arrest situations.
Innovation and service models are shaping growth trends in the region. There is increased emphasis on connected pad technologies that provide real-time status updates and predictive alerts for expiration or readiness, which helps reduce wasted inventory and improve emergency response outcomes. Subscription-based pad replacement services and integrated asset tracking systems are gaining traction among large hospital networks, creating recurring revenue streams and predictable demand.
Europe is likely to be a significant market for defibrillator pads in 2026, due to increased deployment of AEDs, strong healthcare infrastructure, and regulatory emphasis on emergency response readiness. Europe accounts for a substantial share of the global defibrillator pads market, with major markets in Germany, the U.K., France, and Italy, where public access defibrillation programs are expanding alongside clinical demand in hospitals and emergency services. Regulatory frameworks such as the EU Medical Device Regulation (MDR) ensure that pads and related consumables meet stringent safety and performance standards, reinforcing clinical confidence and adoption among healthcare providers.
Market players are focused on broadening their product portfolios to meet diverse needs, from adult to pediatric and multifunction pads. For example, Defibrillator Pads for Lifeline VIEW, PRO & ECG are widely used in European healthcare facilities and public AED programs due to their compatibility with popular AED models and reliable performance in emergencies. Technological enhancements and product availability are shaping market dynamics across Europe. Manufacturers are introducing pads with improved adhesion, extended shelf life, and wider compatibility with different AED brands to address clinician and responder preferences.
The Asia Pacific region is likely to be the fastest-growing region in the defibrillator pads market in 2026, driven by expanding healthcare infrastructure, rising incidence of cardiovascular diseases, and increased public access to AED deployment programs. The region holds a significant share of the global market and is projected to grow rapidly as investments in emergency care rise across China, Japan, India, and ASEAN countries. Governments and health agencies are promoting cardiopulmonary resuscitation (CPR) and AED awareness campaigns, which in turn increase the demand for defibrillator pads used in hospitals and clinics.
Product adoption and competitive presence are shaping regional market dynamics. Leading medical device manufacturers are actively expanding their footprint in the Asia Pacific market with compatible pad solutions tailored to local healthcare needs. For example, Nihon Kohden Corporation’s AED electrode pads are used widely in Japan and other APAC countries, offering reliable performance and interoperability with popular automated external defibrillator models, which enhances emergency response effectiveness in clinical and public settings.
The global defibrillator pads exhibit a moderately fragmented structure, driven by the presence of both large medical technology companies and niche specialists competing across regions and product portfolios. While no single firm completely dominates the market, the top players collectively account for a substantial portion of global sales, underpinned by extensive distribution networks, ongoing product innovation, and strategic alliances.
With key leaders including Philips Healthcare, Zoll Medical Corporation, Medtronic plc, Cardiac Science Corporation, and Nihon Kohden Corporation, competition is anchored in technological differentiation, geographic expansion, and customer-centric strategies. These players compete through continuous R&D investments, strategic partnerships with healthcare providers and EMS organizations, and expansion of global supply chains to improve product availability and performance.
The global defibrillator pads market is projected to reach US$1.4 billion in 2026.
The rising prevalence of cardiovascular diseases, increasing deployment of AEDs in public and healthcare settings, and growing emphasis on rapid emergency response.
The defibrillator pads market is expected to grow at a CAGR of 8.8% from 2026 to 2033.
The expansion of public-access AED programs, growing demand for pediatric and multi-functional pads, adoption of advanced materials with longer shelf life, and increasing penetration in emerging healthcare markets.
Philips, Zoll Medical Corporation, Cardiac Science, Stryker Corporation, and Medline Industries are the leading players.
| Report Attribute | Details |
|---|---|
|
Historical Data |
2020 - 2025 |
|
Forecast Period |
2026 - 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Pad Configuration
By Material Composition
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author