ID: PMRREP32234| 188 Pages | 2 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

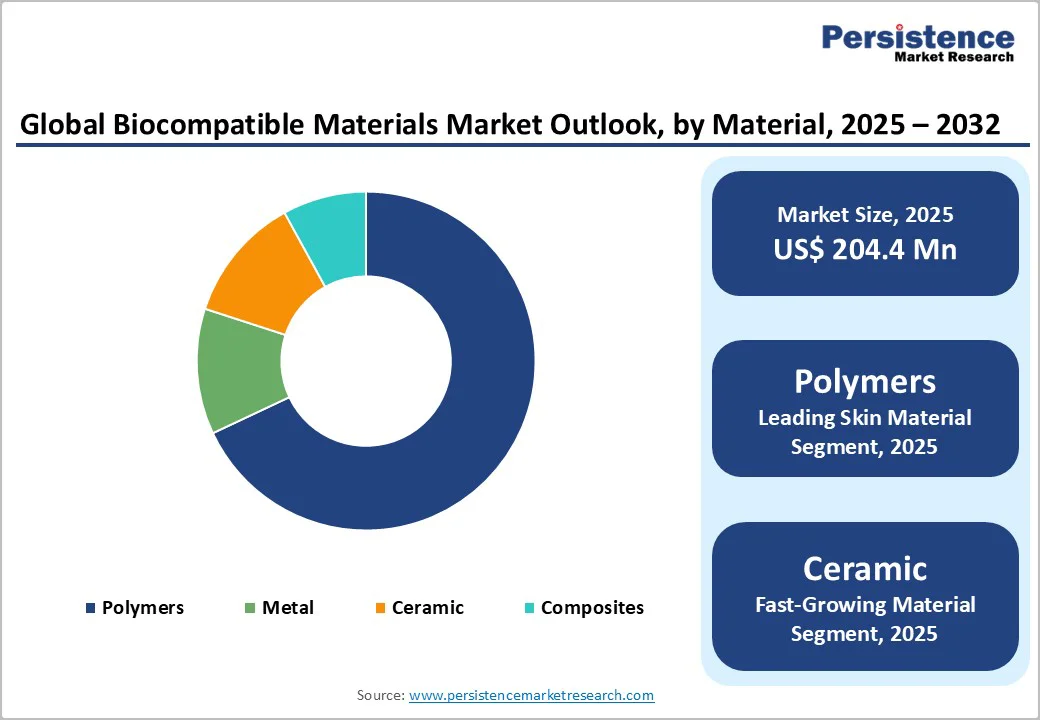
The global biocompatible materials market size is valued at US$ 204.4 million in 2025 and projected to reach US$ 368.9 million, growing at a CAGR of 8.8% during the forecast period from 2025 to 2032.
Biocompatible materials are non-viable materials that do not elicit adverse tissue reactions and are compatible with specific body locations. These non-viable materials are used in medicines that interface with microbiological systems. Biocompatible materials are also useful as implants for bone plates, dental implants, joints, drug delivery, sutures, and surgical or medical instruments for the replacement or restoration of tissues or organs.
Biocompatible materials help to improve people's lives and save millions of lives. Biocompatible materials are expanding in scope due to advanced biomedical technologies, rising demand for cosmetic implants, drug delivery systems, pharmaceutical excipients, tissue engineering, and wound dressings.
| Key Insights | Details |
|---|---|
| Biocompatible Materials Market Size (2025E) | US$ 204.4 Mn |
| Market Value Forecast (2032F) | US$ 368.9 Mn |
| Projected Growth (CAGR 2025 to 2032) | 8.8% |
| Historical Market Growth (CAGR 2019 to 2024) | 7.9% |

Employing medical-grade polymers and biocompatible raw materials, 3D printing and 4D printing technology are currently gaining popularity in the development of patient-specific implants and treatment-specific pharmaceuticals.
Biomaterials are easy to mold, biodegradable, and cost-effective. As a result, medical device manufacturers and biopharmaceutical companies are increasingly adopting technologically developed biocompatible materials.
For instance, DuPont Company presented its novel semi-crystalline materials for 3D applications at RAPID + TCT in April 2019. Advanced semi-crystalline materials will likely increase the 3D printing industry's product segment at RAPID + TCT.
According to a published article on prostheses in the journal of ‘Evidence-Based Complementary and Alternative Medicine’ in 2021, materials for dental implants include calcium phosphate, bioactive ceramics, collagen, fluoride, and titanium nitride. Biomaterials must naturally dissolve; polyhydroxyalkanoates are one example of a biodegradable substance that does not injure tissues or cells by producing bleeding, movement, or peri-implant infections.
Price volatility in biocompatible materials can be attributed to a variety of factors, including economic growth, interest rates, attractiveness, regional availability of domestic raw materials, demand for services and goods, and various political considerations. Manufacturers in the medical biocompatible materials market frequently sign contracts requiring them to purchase the commodity in the agreed-upon quantities and at the agreed-upon prices.
As a result, an unexpected increase in the cost of raw materials and key components is likely to drive up costs, which is expected to hamper manufacturers' operations and, in turn, restrain the growth of the medical biocompatible materials market during the forecast period.
Sometimes, common medical procedures would not be possible without medical devices. Despite being one of the fastest-growing fields, the sale and manufacture of medical devices are governed by a wide range of regulations, which often complicate the field with legal procedures.
The global biocompatible materials market presents significant opportunities as applications expand across emerging medical sectors and geographic regions. Rising demand for advanced tissue engineering, regenerative medicine, and personalized implants is driving the development of novel biocompatible materials, including bioactive ceramics, biodegradable polymers, and nanomaterials.
The growth of minimally invasive surgical procedures and cosmetic interventions, such as breast reconstruction, dental implants, and dermatological treatments, creates further scope for specialized biocompatible solutions.
Emerging markets in the Asia Pacific, Latin America, and the Middle East offer untapped potential due to increasing healthcare investments, expanding medical device infrastructure, and rising awareness of advanced healthcare technologies.
Collaborations between global material manufacturers and local healthcare providers or research institutions can accelerate market penetration. Additionally, 3D and 4D printing technologies enable the development of patient-specific implants, prostheses, and drug delivery systems, enhancing precision and treatment outcomes.
The polymer segment dominated the global biocompatible materials market, accounting for around 68.0% share in 2024. Synthetic polymers have gained significant traction due to their versatile physical, chemical, and biological properties.
Their ability to form three-dimensional matrix structures, exhibit self-reinforcing mechanical behavior, and undergo polymerization and copolymer formation makes them ideal for tissue engineering and regenerative medicine applications.
These properties enable the development of 3D porous scaffolds that support cell growth and tissue repair. In the U.S., polymers such as poly (methyl methacrylate) (PMMA) are widely used in medical devices, including contact lenses, intraocular lenses, bone cement, orthodontic devices, and vascular stents.
Additionally, polymers such as polyamides and polyesters are extensively used as synthetic suture materials and in implant coatings due to their high biocompatibility, flexibility, and resistance to degradation. Continuous advancements in polymer chemistry and the growing adoption of bioresorbable and high-performance polymer materials are expected to strengthen further the dominance of polymers in the global biocompatible materials market.
Medical device manufacturers accounted for approximately 46.2% of the total biocompatible materials market share in 2024. This dominance is attributed to the growing use of biocompatible materials in the design of advanced, patient-specific medical devices.
Manufacturers are increasingly focusing on innovative product designs, such as bioabsorbable cardiac stents reinforced with fine metal meshes that help enhance blood flow by expanding vessel openings. These developments highlight the importance of combining material science with precision engineering to improve device performance and patient outcomes.
Medical devices are categorized based on patient contact duration Class I for non-contact devices, Class II for short-term contact (less than 30 days), and Class III for long-term contact (30 days or more).
The U.S. Food and Drug Administration (FDA) plays a vital role by conducting premarket evaluations of raw materials used in device manufacturing to ensure safety, biocompatibility, and functional reliability. In 2018, the FDA’s Medical Device Safety Action Plan initiated a comprehensive review of metals and other materials used in implants to address patient safety concerns and adverse biological responses linked to long-term exposure.
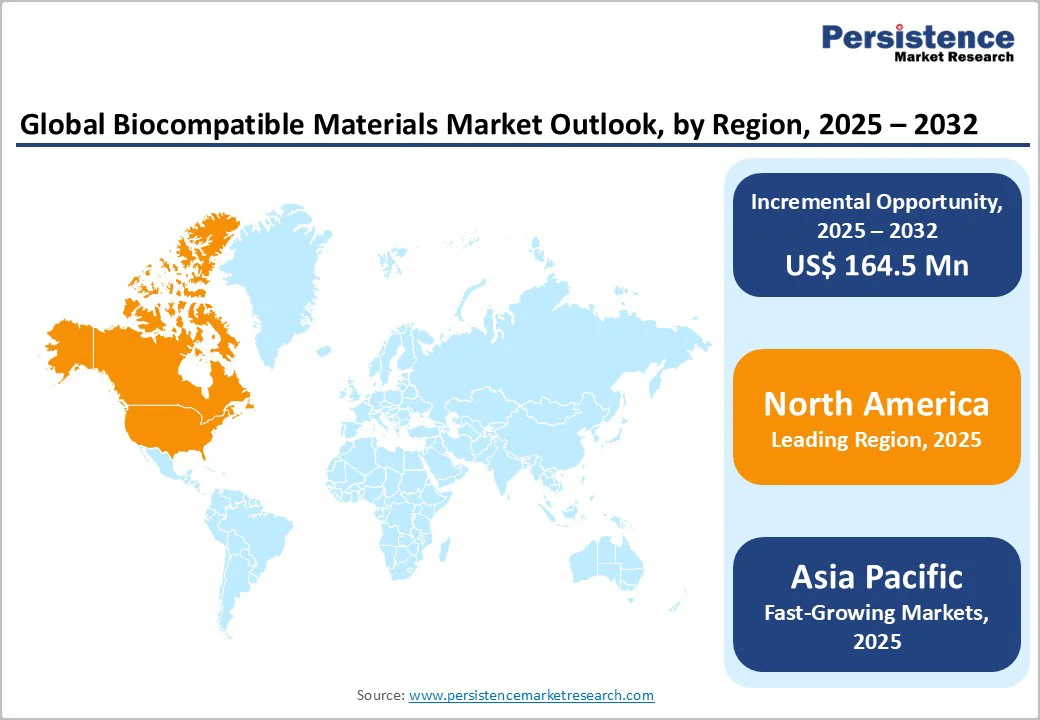
North America, led by the U.S., dominates the global biocompatible materials market, accounting for approximately 33.2% of the total market share in 2024. The region’s growth is driven by a well-established medical device manufacturing industry, advanced healthcare infrastructure, and strong biomaterials research and development initiatives.
Rising adoption of biocompatible materials across applications such as breast augmentation, cosmetic and reconstructive surgery, wound care, and treatments for cancer and cardiovascular diseases is further supporting market expansion.
The increasing use of 3D and 4D printing technologies for patient-specific implants, prosthetics, and tissue engineering is a significant growth factor. Advances in biotechnology, coupled with a high prevalence of chronic diseases and aesthetic procedures, are driving demand for innovative, safe, and customizable biocompatible materials.
Moreover, strategic collaborations, partnerships, and investments by key manufacturers in the region are enhancing product development and commercialization, reinforcing North America’s leadership in the global biocompatible materials market.
The Asia Pacific region is emerging as a high-growth market for biocompatible materials, driven by rising healthcare expenditure, expanding medical infrastructure, and increasing adoption of advanced medical technologies.
Countries like China, India, Japan, and South Korea are at the forefront, investing heavily in innovative applications such as regenerative medicine, cosmetic and reconstructive surgery, and tumor treatments. In China, for example, brain-controlled neuroprostheses and myoelectric prosthetics are being developed to enhance neural function and improve the quality of life for patients with severe motor disabilities.
The adoption of 3D imaging, 3D/4D printing technologies, and miniaturized implants is further driving market growth, enabling minimally invasive procedures, faster recovery, and cost-effective treatments.
Government support, increasing domestic R&D initiatives, and collaborations between local and global companies are facilitating the development and commercialization of advanced biocompatible materials. Rising demand for patient-specific implants, prostheses, and therapeutic devices in emerging economies positions the Asia-Pacific region as the fastest-growing market globally for biocompatible materials.
The global biocompatible materials market is highly competitive, featuring major players focusing on innovation, cost optimization, and technological advancement. Companies are customizing materials to meet specific medical device requirements while maintaining affordability.
Strategic initiatives such as partnerships, mergers, and product launches are common to enhance market presence. Growing investments in 3D printing technologies and medical-grade polymers are fostering innovation across applications like implants and drug delivery systems. Additionally, global supply chain collaborations and regional expansions are intensifying competition and supporting long-term market growth.
The global market is valued at US$ 204.4 Mn in 2025.
Rising demand for patient-specific implants, tissue engineering, and advanced medical devices drives growth in biocompatible materials.
The global market is poised to witness a CAGR of 8.8% between 2025 and 2032.
Expansion in emerging markets, 3D/4D printing applications, regenerative medicine, and nanomaterial-based medical solutions present major opportunities.
Dupont, Ensinger, Stratasys Ltd., Covestro AG, Foster Corporation, Merck KGaA, BASF SE (Exxon Mobil Corporation), Wacker Chemie AG are the key players of biocompatible materials sector.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn and Volume (if Available) |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Material
By Application
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author