ID: PMRREP11935| 210 Pages | 10 Feb 2026 | Format: PDF, Excel, PPT* | Chemicals and Materials

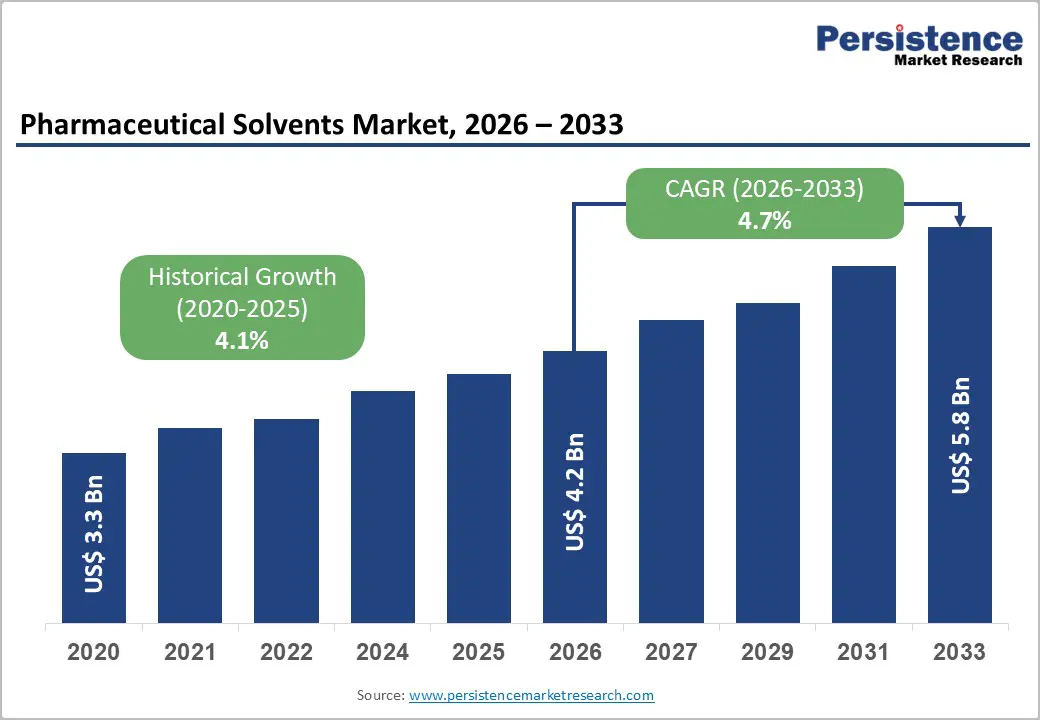
The global pharmaceutical solvents market size is likely to be valued at US$ 4.2 billion in 2026, and is projected to reach US$ 5.8 billion by 2033, growing at a CAGR of 4.7% during the forecast period 2026−2033. The market is set to undergo stable expansion driven by sustained growth in pharmaceutical manufacturing intensity and rising complexity of drug development pipelines. Increasing prevalence of chronic and infectious diseases continues to elevate demand for both small-molecule and complex drug formulations, directly increasing solvent consumption across synthesis, purification, and formulation processes. Expansion of generic drug manufacturing across regulated and semi-regulated markets reinforces volume demand for cost-efficient and compliant solvent systems. Regulatory emphasis on product quality, impurity control, and reproducibility strengthens adoption of high-purity pharmaceutical-grade solvents across production stages. Technological integration within pharmaceutical manufacturing, including continuous processing and automated purification platforms, raises the need for solvents with consistent physicochemical profiles and predictable performance.
| Report Attribute | Details |
|---|---|
|
Pharmaceutical Solvents Market Size (2026E) |
US$ 4.2 Bn |
|
Market Value Forecast (2033F) |
US$ 5.8 Bn |
|
Projected Growth (CAGR 2026 to 2033) |
4.7% |
|
Historical Market Growth (CAGR 2020 to 2025) |
4.1% |
Expansion of Pharmaceutical Manufacturing Capacity and Formulation Complexity
Rapid scale-up of pharmaceutical manufacturing capacity transformed solvent demand patterns across synthesis, purification, and formulation stages. Large-volume production lines require consistent solvent quality, tighter impurity thresholds, and assured supply continuity to maintain batch integrity and regulatory compliance. Capacity expansion increases parallel processing of active pharmaceutical ingredients (APIs) and intermediates, raising cumulative solvent consumption per facility. Continuous manufacturing and multipurpose plants intensify solvent turnover, as frequent product changeovers require validated cleaning, flushing, and recovery cycles. Investment in new plants across Asia Pacific and Europe reinforces this dynamic, with solvent selection becoming a strategic procurement decision linked to yield optimization, waste minimization, and operational efficiency rather than a purely auxiliary input.
Rising formulation complexity elevates solvent relevance within modern drug development and commercialization. Advanced dosage forms such as controlled-release systems, biologic–small molecule combinations, and poorly soluble compounds rely on precise solvent systems to ensure stability, bioavailability, and manufacturability. Solvents function as enablers of particle size reduction, polymorph control, and uniform dispersion during formulation and scale-up. Increasing regulatory scrutiny on product consistency emphasizes solvent reproducibility and documentation across global supply chains. According to the US Food and Drug Administration (FDA), 55 novel drugs received approval in 2023, reflecting a sustained pipeline of complex therapies demanding sophisticated formulation strategies and specialized solvent use as technical inputs supporting performance, compliance, and commercial viability.
Volatility in Raw Material Availability and Supply Chain Constraints
Supply of raw materials for pharmaceutical solvents remains unpredictable, affecting production efficiency and operational planning. Many solvents rely on petrochemical derivatives, specialty chemicals, or plant-based feedstock, which experience seasonal, geopolitical, and market-driven fluctuations. Limited sourcing options for high-purity intermediates create risk of supply disruptions, affecting manufacturing timelines. Transportation bottlenecks, port delays, and regional production restrictions further amplify uncertainty in procurement cycles. Price volatility from these supply challenges increases production costs, forcing companies to either absorb higher expenses or transfer them along the value chain, affecting competitiveness.
Operational continuity faces constraints as pharmaceutical manufacturers maintain compliance with stringent quality standards. Solvent substitution presents challenges, as minor deviations in purity or chemical composition compromise drug formulation stability and regulatory adherence. Long lead times for specialty solvents limit response to sudden shifts in demand or supply interruptions, affecting production scheduling and inventory strategies. Multi-country supply chains magnify delays when local disruptions occur. Cost escalation, regulatory sensitivity, and logistical uncertainty restrain operational efficiency, slowing growth initiatives and investment in production expansion. Risk mitigation strategies, including diversified sourcing and inventory optimization, reduce exposure, although structural supply chain constraints persist.
Growth of Pharmaceutical Manufacturing in Emerging Economies
Rapid expansion of pharmaceutical manufacturing in emerging economies creates significant demand for high-quality solvents used in drug synthesis, formulation, and production processes. Low production costs, supportive regulatory frameworks, and access to skilled labor encourage multinational and local companies to establish manufacturing facilities in these regions. This expansion requires a steady supply of solvents for various applications, including API production, excipient preparation, and cleaning processes. Industrial diversification in these regions drives investments in modern manufacturing technologies, which rely on solvents with consistent purity and performance. Solvent suppliers can capitalize on volume growth, long-term supply agreements, and partnerships with manufacturers seeking reliable chemical inputs.
Emerging economies witness increased domestic and regional demand for pharmaceutical products driven by rising healthcare access, urbanization, and chronic disease prevalence. This growth prompts manufacturers to scale up production capacities and adopt continuous manufacturing techniques, which require specialized solvents tailored to advanced production methods. Investment in research and development hubs in these regions creates opportunities for solvent innovation, including high-purity, environmentally friendly, and recyclable variants. Solvent manufacturers positioned to offer technical support, regulatory compliance assistance, and custom solutions strengthen client relationships and secure strategic contracts.
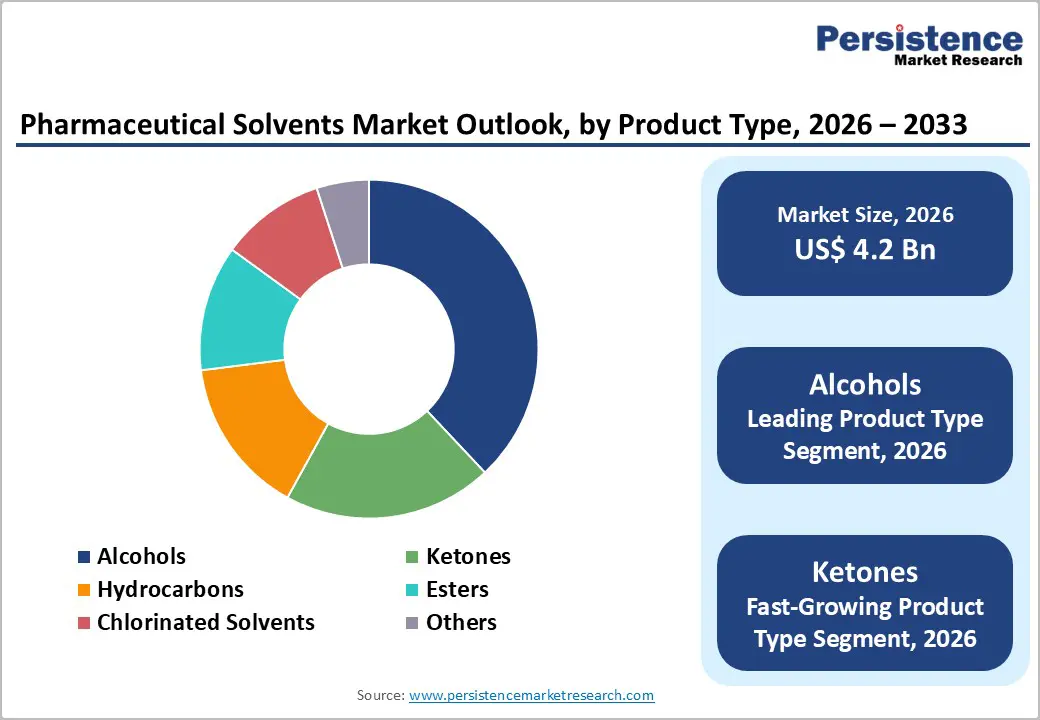
Product Type Insights
Alcohols are anticipated to secure around 38% of the pharmaceutical solvents market revenue share in 2026, reflecting widespread applicability across synthesis, formulation, and cleaning operations. Ethanol and isopropyl alcohol demonstrate high solvency, favorable evaporation characteristics, and established regulatory acceptance. Their chemical versatility enables use in a broad range of pharmaceutical processes, including extraction, purification, and intermediate preparation. Extensive integration into API synthesis and various formulation processes ensures steady consumption across manufacturers. Compatibility with sterilization protocols and cleaning operations enhances operational efficiency in production facilities. Robust supplier networks, global availability, and streamlined logistics strengthen procurement reliability, reducing risk of supply disruptions and supporting sustained market adoption.
Ketones are expected to be the fastest-growing segment during the 2026–2033 forecast period, propelled by rising adoption in complex synthesis pathways and controlled-release formulations. Their favorable polarity profiles, high solvency, and chemical stability allow efficient processing of specialized intermediates and sensitive APIs. Expanding use in research and development laboratories, pilot production, and continuous manufacturing contributes to growth acceleration. Regulatory familiarity, along with improvements in recovery and recycling technologies, enhances cost efficiency while reducing environmental impact. Pharmaceutical companies increasingly rely on ketones for advanced drug development, making them critical for next-generation formulations and supporting sustained adoption across emerging and established markets.
Application Insights
API manufacturing is poised to dominate with a forecasted market share of 45% in 2026, powered by multi-step synthesis processes requiring repeated solvent utilization. Strict purity standards and rigorous impurity control protocols drive high solvent demand at every stage, from intermediate preparation to final API isolation. Expansion of generic and specialty drug pipelines supports continuous production, ensuring stable consumption of solvents. Advanced production technologies, including continuous flow synthesis and automated purification, increase process efficiency but maintain solvent dependency. Strong regulatory oversight emphasizes validated cleaning and solvent recovery, reinforcing the critical role of solvents in achieving consistent product quality and operational reliability.
Research & development is likely to be the fastest-growing segment from 2026 to 2033, fueled by expanding drug discovery pipelines and increased investment in early-stage development. Solvents are extensively used in high-throughput screening, formulation testing, stability studies, and scale-up experiments, supporting diverse experimental requirements. Adoption of digital laboratory platforms and automation improves process reproducibility while increasing solvent throughput. Growth in biotechnology, personalized medicine, and novel delivery systems drives demand for specialized and high-purity solvents. Laboratory initiatives emphasizing environmentally friendly practices, solvent recycling, and process optimization further shape consumption patterns, strengthening solvent relevance across research and early-stage development activities.
End-User Insights
Pharmaceutical companies are slated to be the leading segment with a projected 52% of the pharmaceutical solvents market share in 2026 due to integrated manufacturing operations and large-scale production capacity. High-volume production lines, including API synthesis, formulation, and packaging, require consistent and reliable solvent supply. Established compliance frameworks, such as Good Manufacturing Practices (GMP) and environmental regulations, ensure solvent use aligns with quality and safety standards. Long-term supplier contracts and strategic procurement partnerships support uninterrupted operations. Advanced process technologies, including continuous manufacturing and automated cleaning systems, further reinforce solvent demand, enabling efficient production while maintaining regulatory and quality compliance across diverse manufacturing environments.
Biotechnology companies are anticipated to be the fastest-growing segment from 2026 to 2033, fueled by expansion of biologics, specialty drugs, and novel delivery systems. Complex synthesis and purification processes for recombinant proteins, monoclonal antibodies, and cell therapies require high-purity solvents at multiple stages. Precision in solvent selection directly impacts product stability, yield, and efficacy, driving intensive use across development and manufacturing activities. Emerging biotechnology hubs and increased investment in early-stage research amplify solvent consumption. Adoption of scalable, modular manufacturing platforms and integration of automated purification technologies support growth, while environmentally conscious solvent recovery initiatives enhance operational efficiency, reinforcing solvent relevance across the biotechnology sector.
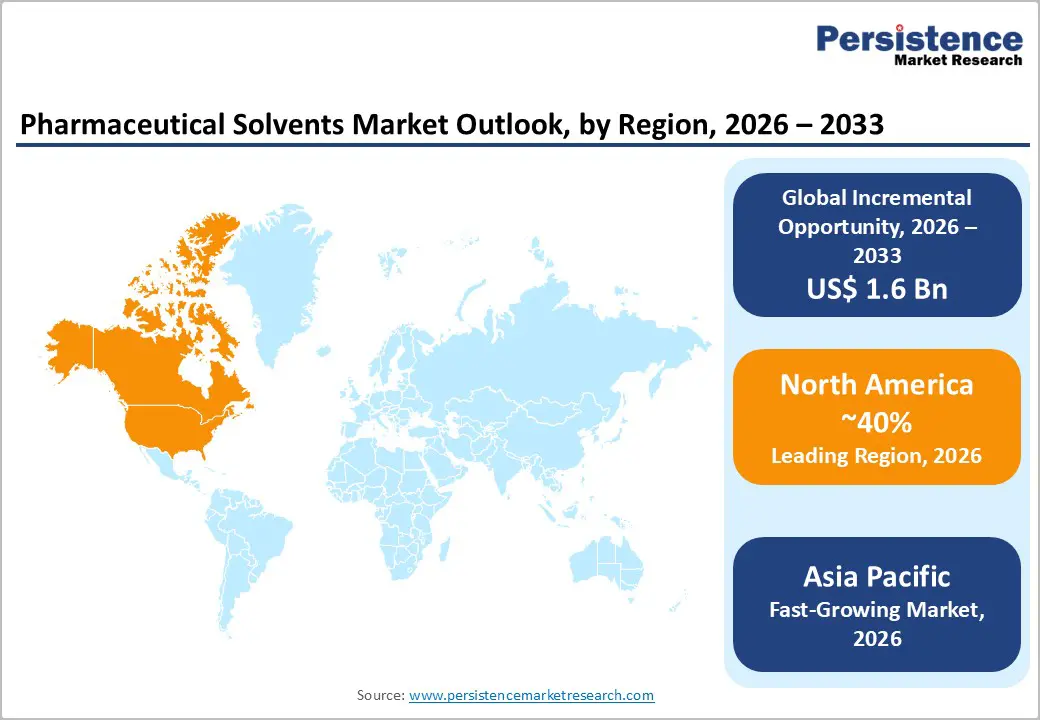
North America Pharmaceutical Solvents Market Trends
North America is anticipated to secure approximately 40% of the pharmaceutical solvents market sales in 2026, reflecting extensive pharmaceutical manufacturing capacity and well-established research infrastructure. High concentration of multinational pharmaceutical manufacturers and contract development and manufacturing organizations (CDMOs) drives sustained solvent demand across synthesis, formulation, and cleaning operations. Stringent regulatory oversight and compliance standards for purity, residual solvents, and environmental impact enforce high-quality solvent utilization at multiple production stages. Continuous manufacturing adoption, high-throughput screening, and biologics production intensify solvent consumption, particularly for high-purity alcohols, ketones, and specialty solvents. Established procurement networks, global supplier integration, and long-term supply agreements ensure consistent solvent availability, mitigating operational risks and supporting uninterrupted production pipelines.
Advanced investment in innovation and technology further reinforces dominance. Expansion of biologics, cell and gene therapies, and complex APIs increases reliance on precision synthesis and specialized purification processes, enhancing solvent intensity per production unit. Research hubs with automated laboratories and digital platforms improve experimental reproducibility, generating higher volumes of solvent consumption in early-stage development. Environmental sustainability initiatives, including solvent recovery, recycling, and energy-efficient processes, create opportunities for high-performance solvent adoption without compromising compliance standards. Consolidation of pharmaceutical operations, coupled with integration of modular production facilities and scalable manufacturing platforms, strengthens operational efficiency while driving continuous demand for solvents across multiple production and R&D applications.
Europe Pharmaceutical Solvents Market Trends
In Europe, the market for pharmaceutical solvents is foreseen to demonstrate steady growth during the 2026-2033 forecast period, supported by mature pharmaceutical manufacturing infrastructure and strong regulatory frameworks. High concentration of multinational pharmaceutical companies and contract manufacturing organizations ensures consistent demand for solvents across synthesis, formulation, and cleaning processes. Focus on quality standards, environmental compliance, and safety protocols drives preference for high-purity solvents, particularly alcohols, ketones, and specialty reagents. Established supply chains, advanced logistics networks, and robust procurement systems enable reliable solvent availability, supporting uninterrupted production operations. Expansion of biologics, controlled-release formulations, and complex APIs increases process complexity, reinforcing solvent usage across multiple stages of manufacturing and development activities.
Innovation and sustainability initiatives further shape market dynamics. Widening adoption of green chemistry practices, solvent recovery systems, and energy-efficient production technologies promotes efficient solvent utilization while aligning with stringent regulatory expectations. Research and development investments, particularly in precision medicine, gene and cell therapies, and novel delivery systems, generate higher solvent consumption in experimental and early-stage development activities. Collaboration between manufacturers, technology providers, and suppliers enhances technical support, ensuring compliance with evolving environmental and quality standards. Focus on process optimization, continuous manufacturing, and modular production platforms strengthens operational efficiency while maintaining high solvent demand. Strategic positioning of companies in high-value therapeutic segments supports long-term stability, making the market resilient and technologically advanced.
Asia Pacific Pharmaceutical Solvents Market Trends
Asia Pacific is forecasted to be the fastest-growing market for pharmaceutical solvents between 2026 and 2033, driven by rapid expansion of pharmaceutical and biotechnology manufacturing capabilities. Investment in large-scale production facilities for generic drugs, specialty pharmaceuticals, and biologics supports rising solvent demand across synthesis, purification, and formulation processes. Lower operational costs and availability of skilled technical labor attract multinational companies to establish manufacturing and research operations, generating high volumes of solvent consumption. Growing presence of CDMOs facilitates scaling of production capacities, further increasing reliance on high-purity solvents. Growth in domestic and regional healthcare access, combined with rising prevalence of chronic and lifestyle diseases, stimulates demand for diversified therapeutic portfolios, strengthening production pipelines and driving consistent solvent usage.
Adoption of advanced manufacturing technologies accelerates market growth by increasing process efficiency and solvent intensity. Continuous production platforms, automated purification systems, and precision-controlled synthesis methods support production of complex APIs and biologics, elevating demand for specialized solvents such as ketones, alcohols, and high-purity reagents. Rising investment in research and development hubs amplifies early-stage experimental solvent consumption, particularly in high-throughput screening, formulation optimization, and pilot-scale studies. Regulatory frameworks promoting industrial growth and quality compliance encourage adoption of environmentally friendly solvent recovery and recycling processes, supporting sustainable operations. Strategic partnerships between manufacturers, suppliers, and technology providers enhance technical support and ensure consistent supply, enabling rapid market expansion.
The global pharmaceutical solvents market is characterized by a moderately fragmented structure, where global chemical producers and specialized solvent suppliers compete across product quality, regulatory compliance, and distribution capabilities. Leading players, including BASF, Merck KGaA, Eastman Chemical Company, Dow Inc., and LyondellBasell Industries Holdings B.V., maintain a significant share by leveraging extensive manufacturing capacities, established supply chains, and global reach. These companies emphasize production of high-purity solvents suitable for synthesis, formulation, and cleaning applications, ensuring alignment with stringent quality and environmental standards.
Competitive positioning in the market prioritizes regulatory alignment, consistent supply reliability, and technological innovation. Companies invest in research and development to produce specialty solvents for advanced drug development, biologics manufacturing, and high-throughput laboratory applications. Adoption of environmentally sustainable practices, such as solvent recovery, recycling, and energy-efficient production, differentiates key players while meeting regulatory and industry expectations. Long-term supply agreements and collaborative partnerships with pharmaceutical manufacturers enhance operational continuity and client trust. Emphasis on technical service, customer support, and compliance consulting further reinforces market position, allowing leading players to sustain growth in a dynamic, innovation-driven environment.
Key Industry Developments
The global pharmaceutical solvents market is projected to reach US$ 4.2 billion in 2026.
Expansion of pharmaceutical manufacturing capacity and regulatory enforcement of high-purity standards are driving the market.
The market is poised to witness a CAGR of 4.7% from 2026 to 2033.
Growth of pharmaceutical manufacturing in emerging economies and increasing adoption of sustainable, green solvents present key market opportunities.
Some of the key market players include BASF, Merck KGaA, Eastman Chemical Company, Dow Inc., and LyondellBasell Industries Holdings B.V.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 – 2025 |
|
Forecast Period |
2026 – 2033 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Application
By End-User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author