ID: PMRREP3197| 188 Pages | 5 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

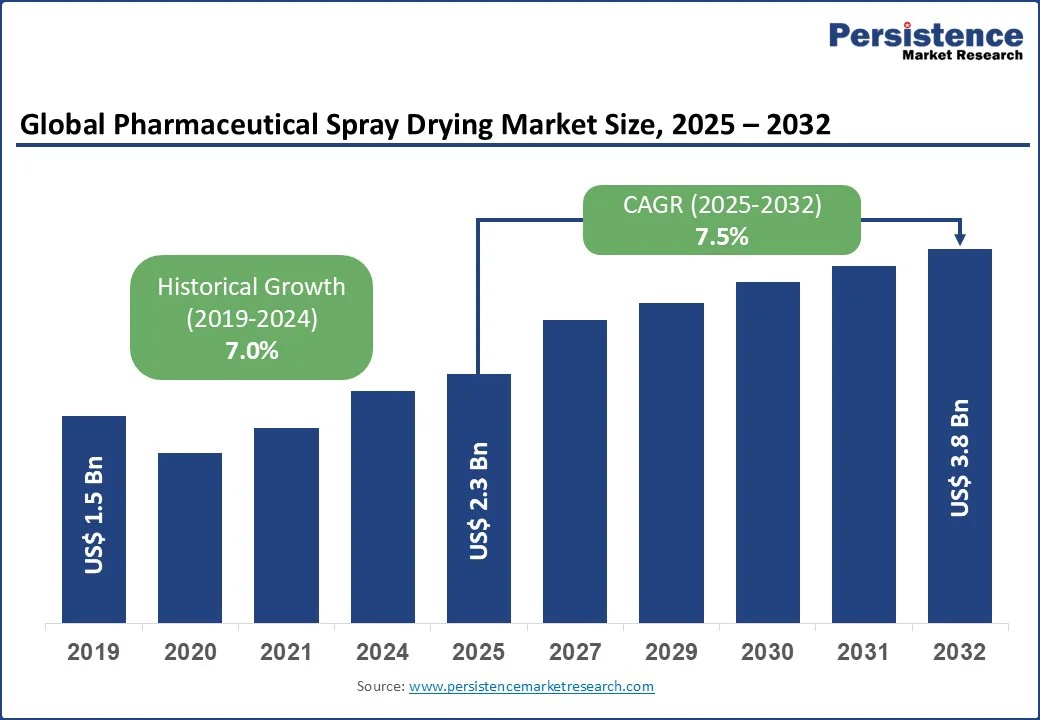
The global pharmaceutical spray drying market Size is projected to reach US$2.3 Bn in 2025 and is expected to grow to US$3.8 Bn by 2032, with a CAGR of 7.5% during the forecast period 2025 to 2032.
The pharmaceutical spray drying industry is driven by the growing need for advanced drug delivery solutions, including amorphous solid dispersions for bioavailability enhancement and dry powder inhalers for respiratory therapies.
Key Industry Highlights
|
Global Market Attribute |
Key Insights |
|
Pharmaceutical Spray Drying Market Size (2025E) |
US$2.3 Bn |
|
Market Value Forecast (2032F) |
US$3.8 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.5% |
|
Historical Growth (CAGR 2019 to 2024) |
7.0% |
Rising adoption in biologics manufacturing, protein-based therapeutics, and vaccine development is further accelerating demand. Technological innovations such as closed-loop systems, supercritical fluid spray drying, and process analytical tools are shaping the market, addressing challenges related to solvent recovery, containment, and regulatory compliance.
The pharmaceutical spray drying market is driven by the escalating need for enhanced drug solubility and bioavailability, particularly for complex formulations addressing chronic diseases, biologics, and personalized medicine. Spray drying is a cornerstone of pharmaceutical manufacturing, enabling the production of powders, granules, and suspensions with improved solubility for poorly soluble APIs, which form a significant portion of modern drug pipelines.
This technique facilitates the creation of amorphous solid dispersions, significantly enhancing bioavailability for drugs targeting conditions such as cancer, diabetes, cardiovascular diseases, and respiratory disorders, aligning with health benefits.
The growing demand for inhalable drug formulations, such as dry powder inhalers for respiratory conditions, drives the need for spray-dried powders with precise particle sizes, ensuring effective lung delivery and improved patient outcomes.
The rise in biologics production, including vaccines, monoclonal antibodies, and protein-based therapeutics, further fuels demand, as spray drying ensures stability for heat-sensitive materials, making it critical for vaccine production and biologics spray drying. Advancements in equipment, such as closed-loop spray dryers and supercritical fluid spray drying, offer precise control over particle morphology and size distribution, supporting spray technology innovations for personalized medicine.
The pharmaceutical spray drying market faces significant challenges due to the high capital and operational costs of pharmaceutical equipment, limiting adoption in smaller facilities and emerging markets. Closed-loop spray dryers, critical for biologics spray drying and handling flammable solvents, incorporate advanced components such as high-efficiency nozzles, automated control systems, and nitrogen-based drying processes, resulting in substantial capital investment.
Similarly, supercritical fluid spray drying systems, used for inhalable drug formulations and vaccine production, are costly due to their sophisticated technology and specialized materials, making them less accessible for smaller pharmaceutical manufacturing firms and diagnostic laboratories.
Operational costs, including energy consumption, maintenance, and the need for controlled environments to ensure cGMP compliance, further drive expenses, impacting the scalability of drying applications. The high cost of raw materials, such as APIs and excipients, adds to the financial burden, particularly for biologics production and chemicals spray drying, limiting affordability in price-sensitive regions. Continuous upgrades to meet evolving regulatory standards, such as those set by the FDA and European
The pharmaceutical spray drying market offers significant growth opportunities through the expansion of biologics production and inhalable drug formulations, driven by API spray drying innovations and rising demand for advanced therapeutics. The increasing focus on biologics, including vaccines, monoclonal antibodies, protein-based therapeutics, and gene therapies, creates substantial potential, as spray drying ensures stability for heat-sensitive compounds, supporting the health benefits of biologics spray drying and pharmaceutical spray technology.
This technology is critical for producing stable powders and granules that maintain the efficacy of biologics, enhancing vaccine production and chronic disease treatment. The rising popularity of inhalable drug formulations, such as dry powder inhalers for respiratory conditions such as asthma and COPD, drives demand for spray-dried powders with precise particle sizes and aerodynamic properties, achievable through supercritical fluid spray drying and enhanced spray drying, aligning with API spray drying trends.
Emerging markets, with rapidly developing healthcare infrastructure and increasing investments in pharmaceutical manufacturing, offer untapped potential for spray drying systems, as governments prioritize modernizing diagnostic laboratories and hospital diagnostics.
Closed-loop spray dryers hold a 60% market share in 2025, driven by their ability to handle flammable solvents and oxygen-sensitive materials, making them ideal for biologics atomization drying and pharmaceutical manufacturing. Their nitrogen-based drying process ensures safety and product stability, enhancing health benefits.
Open-loop spray dryers are the fastest-growing, fueled by increasing demand for inhalable drug formulations and biologics production. The segment’s growth is also bolstered by continuous innovations in spray drying technology, including enhanced atomization methods, improved thermal efficiency, and better process control systems.
Powders are expected to hold a 50% market share in 2025, driven by their widespread use in inhalable drug formulations and oral drug delivery systems, which offer enhanced solubility, stability, and patient compliance in pharmaceutical manufacturing.
Granules are the fastest-growing, fueled by demand for biologics, spray drying and vaccine production, aligning with pharmaceutical aerosol drying consumer preferences. These innovations position granules as a preferred format for next-generation biologics and vaccines
Conventional spray drying leads with a 55% market share in 2025, valued for its cost-effectiveness and established infrastructure in pharmaceutical manufacturing and food and beverage spray drying. This dominance is largely due to the widespread availability of conventional misting dryers, which have been a standard in industrial drying processes for decades.
Supercritical fluid spray drying is the fastest-growing, driven by its precision in producing nanoparticles for inhalable drug formulations and biologics production. The increasing demand for advanced respiratory therapies, including treatments for asthma, COPD, and targeted pulmonary delivery for systemic diseases, has amplified the need for technologies such as SCFSD.
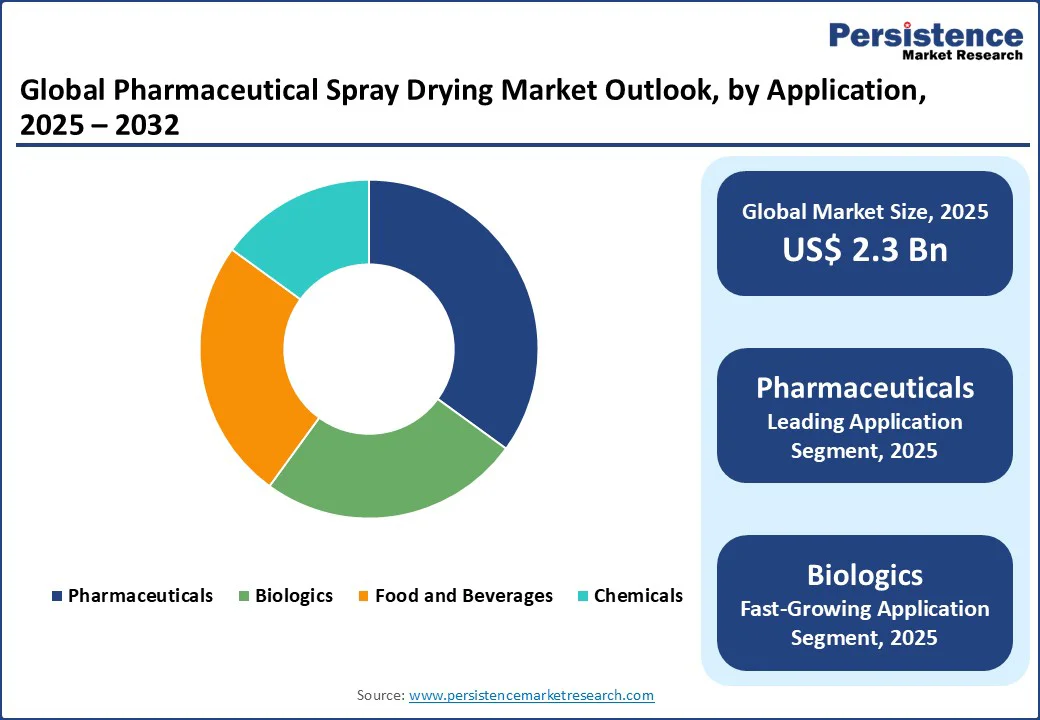
Pharmaceuticals lead with a 45% market share in 2025, driven by the need for bioavailability enhancement in oral drug delivery systems and inhalable drug formulations. Atomization drying has become a preferred technique for creating amorphous solid dispersions (ASDs), which significantly improve the solubility and absorption of poorly water-soluble drugs—one of the biggest challenges in oral dosage form development.
Biologics are the fastest-growing, fueled by demand for vaccine production and protein-based therapeutics. Misting drying is increasingly used for biologics due to its ability to produce stable, dry formulations of sensitive biomolecules without significant loss of structural integrity or activity.
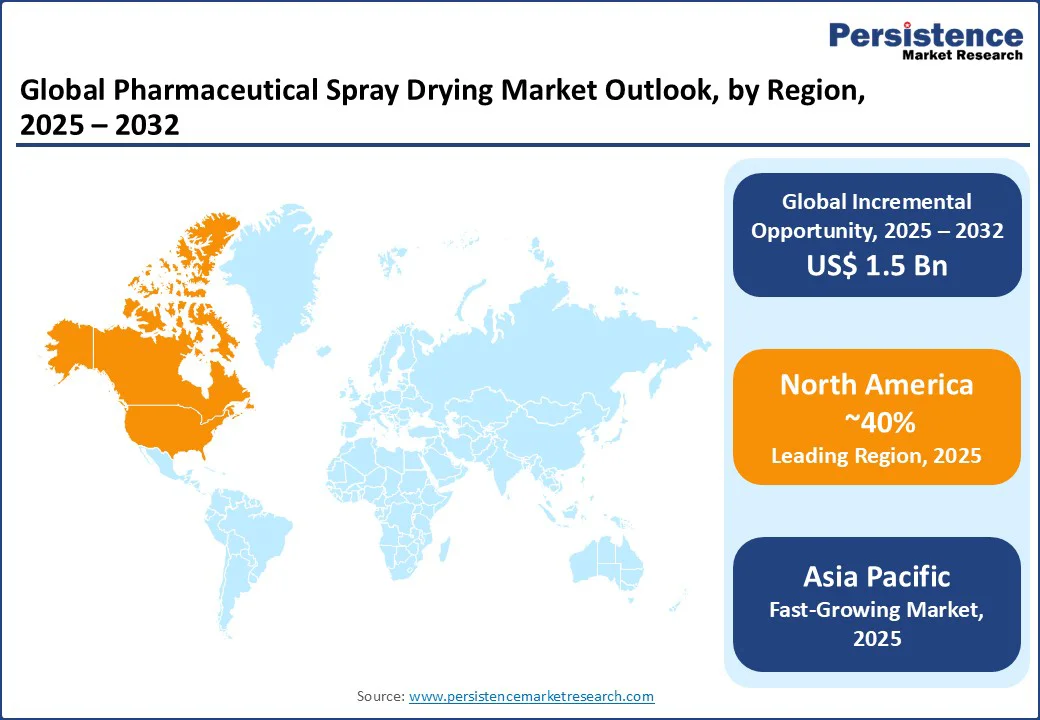
North America holds a 40% global market share in 2025, led by the U.S. Advanced pharmaceutical infrastructure, significant R&D investments, and a high prevalence of chronic diseases, such as cancer and diabetes, drive the region’s dominance. The U.S. market thrives on spray drying equipment, particularly closed-loop spray dryers, for biologics spray drying and inhalable drug formulations.
R&D spending, exceeding US$100 Bn annually in the pharmaceutical sector, fosters drying innovations such as supercritical fluid misting drying, enhancing bioavailability, and vaccine production. The FDA’s regulatory framework ensures cGMP compliance, boosting the health benefits of pharmaceutical spray technology and drug delivery efficiency. The rise of atomization spray drying e-commerce enhances access to spray drying systems for diagnostic laboratories and hospital diagnostics.
Europe holds a 28% global market share in 2025, led by Germany, the UK, and France. Advanced pharmaceutical industries and a focus on biologics spray drying drive the region’s growth. Germany leads in pharmaceutical manufacturing, with diagnostic laboratories adopting closed-loop spray dryers for inhalable drug formulations.
The UK emphasizes chronic disease treatment, with atomization spray drying applications supporting oral drug delivery systems and vaccine production. France drives personalized medicine with supercritical fluid spray drying for bioavailability enhancement.
The European Medicines Agency’s cGMP compliance standards ensure quality, boosting pharmaceutical spray technology brands such as Buchi Labortechnik AG and GEA Group. The rise of e-commerce enhances misting drying systems distribution, aligning with market trends.
Asia Pacific is the fastest-growing region, with a 23% global market share in 2025. China, Japan, and India lead due to rapid healthcare modernization and pharmaceutical manufacturing investments. China’s growth is fueled by government support for biologics production, with atomization spray drying equipment adopted for inhalable drug formulations and vaccine production.
Japan’s focus on precision medicine drives supercritical fluid spray drying for oral drug delivery systems. India’s market is expanding due to rising healthcare access and chronic disease prevalence, which in turn boosts demand for pharmaceutical spray technology in hospital diagnostics.
Companies such as Yamato Scientific America, Inc. and Hosokawa Micron Ltd lead with pharmaceutical spray technology innovations, supported by e-commerce. The region’s growth is driven by chronic disease treatment and health benefits, positioning the Asia Pacific as a hub for pharmaceutical misting technology trends.
The global pharmaceutical spray drying market's Competitive Landscape is characterized by the presence of both established and emerging players. Established players have a strong market presence and a wide product portfolio, while emerging players are focusing on niche markets and offering innovative solutions. BCHI Labortechnik AG, a leading company, offers a comprehensive range of spray drying systems and accessories.
The company's products are known for their high quality, reliability, and efficiency. GEA Group AG is a competitor that is the leading provider of process engineering solutions. The company's spray drying systems are designed to meet the specific requirements of the pharmaceutical industry.
The pharmaceutical spray drying market is projected to reach US$ 2.3 Bn in 2025.
Demand for enhanced drug solubility and bioavailability fuels pharmaceutical spray drying demand in biologics production.
The pharmaceutical spray drying market grows at a CAGR of 7.5% from 2025 to 2032, reaching US$ 3.8 Bn by 2032.
Expansion in biologics production and inhalable drug formulations drives pharmaceutical spray drying growth.
Key players include GEA Group, SPX FLOW, Inc., Buchi Labortechnik AG, and Hosokawa Micron Ltd.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product Type
By Dosage
By Technology
By Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author