ID: PMRREP24067| 210 Pages | 2 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

The global pharmaceutical isolators market size is valued at US$683.1 million in 2025 and projected to reach US$948.6 million, growing at a CAGR of 4.8% during the forecast period from 2025 to 2032.
The pharmaceutical isolators industry is expanding steadily as biopharma companies prioritize advanced sterile manufacturing and high-containment environments. Isolators provide a closed, controlled system that ensures product integrity, operator safety, and regulatory compliance, especially for aseptic processing and potent compound handling. Rising production of biologics, cytotoxic drugs, and personalized therapies is accelerating adoption, alongside stricter GMP guidelines that favor isolator-based systems over traditional cleanrooms.
| Key Insights | Details |
|---|---|
|
Pharmaceutical Isolators Market Size (2025E) |
US$683.1Mn |
|
Market Value Forecast (2032F) |
US$948.6 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
4.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
4.2% |
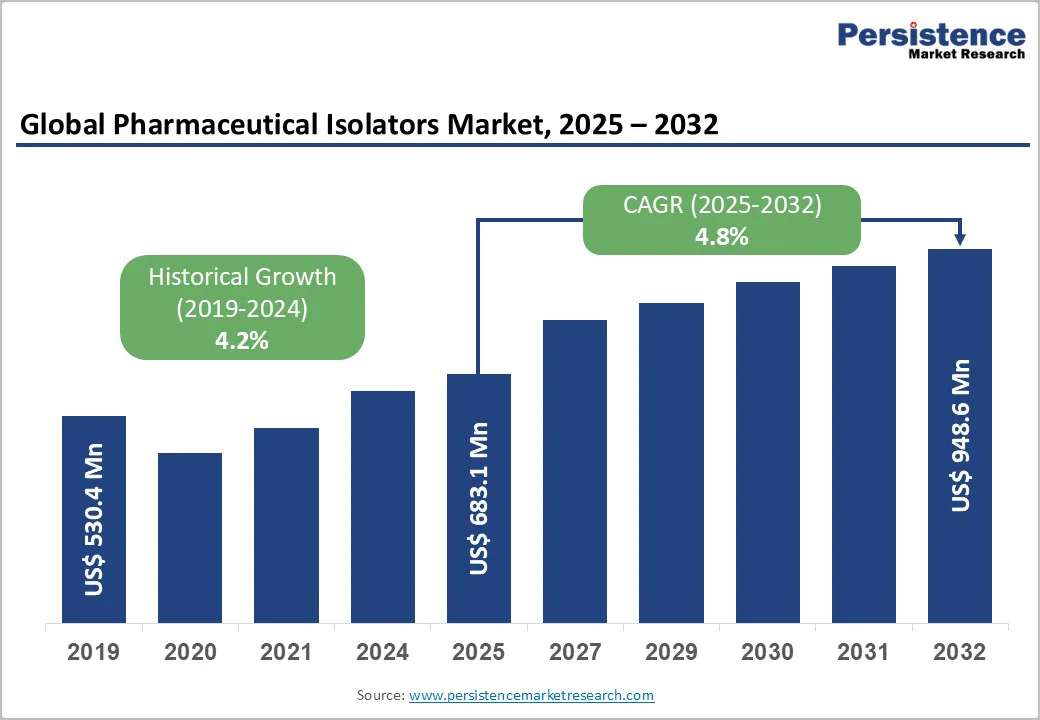
Most pharmaceutical isolators are created to meet the requirements of manufacturing or research facilities. Increased collaborative relationships and a thorough product approval process drive market expansion. Another important factor influencing market growth is the low operating expenses of pharmaceutical isolators. Pharmaceutical businesses can purchase isolators at a fraction of the cost of typical clean rooms from reliable suppliers.
An operator can contaminate a spot that has been thoroughly cleaned and disinfected. Conversely, pharmaceutical isolators reduce contamination, ensuring that only production materials and medicines are in contact with the full management system in a bio-decontaminated environment.
Moreover, the expansion of the biotechnology industry has driven demand for pharmaceutical isolators. The biotechnology sector has advanced significantly and is now among the largest in the world. Both large biotech organizations such as Pfizer, Novartis, Abbott, and Biogen Inc., as well as smaller biotech firms, are scattered across the medical industry in areas covering medication development, genomics, biofuels, and food products. With time, biotechnology has also entered the biopharmaceutical industry, aiding these businesses in their R&D efforts.
Furthermore, important developments in pharmaceutical isolators, such as improved mobility, greater compatibility with active pharmaceutical ingredients (APIs), and enhanced sterility assurance, will drive market growth in the future. These elements have worked together to accelerate the market for pharmaceutical isolators.
Pharmaceutical isolators play an important role in healthcare facilities, delivering safety and efficiency in pharma-biotech companies. Pharmaceutical isolators have superior qualities yet come with a few drawbacks.
In future years, the pharmaceutical isolators sector will undoubtedly grow rapidly. However, several obstacles may prevent the market from reaching its full potential. Because pharmaceutical isolators are expensive to install and maintain, the market may not expand as quickly as it might have otherwise.
Another obstacle to the expansion of pharmaceutical isolator technology is the limited adoption of restricted access barrier systems (RABS) in healthcare manufacturing, which prevent contamination of sterile products by minimizing contact with operating personnel. These systems require larger operating areas.
Thus, higher installation costs and requirements for appropriate space will be restrictive factors for market growth, imposing additional infrastructure and operational overhead costs.
The rise of autologous cell therapies, gene-modified treatments, and personalized oncology regimens is generating strong demand for isolators tailored to small-batch, patient-specific manufacturing. These therapies require extremely sterile, tightly controlled environments with rapid turnaround times. Compact, modular isolators offer an ideal solution by enabling flexible, on-demand processing without large facility footprints. Their customizable chambers, automated decontamination cycles, and seamless integration with closed processing systems support high sterility assurance for individualized batches. As decentralized and point-of-care manufacturing models grow, purpose-built isolators for small-volume aseptic workflows will become a critical infrastructure component, opening new growth avenues for manufacturers.
Closed isolators account for the highest share in the pharmaceutical isolators market due to their superior sterility assurance and contamination control compared to open systems. These fully enclosed, pressure-controlled units minimize human intervention, reducing the risk of microbial contamination during critical processes such as aseptic filling, sterility testing, and handling of high-potency drugs. Strict regulatory requirements from the FDA, EMA, and PIC/S increasingly favor closed systems for compliance with Good Manufacturing Practices (GMP). Additionally, closed isolators support automation, modular integration, and efficient handling of biologics, vaccines, and cytotoxic compounds, making them the preferred choice for pharmaceutical and biotech manufacturers globally.
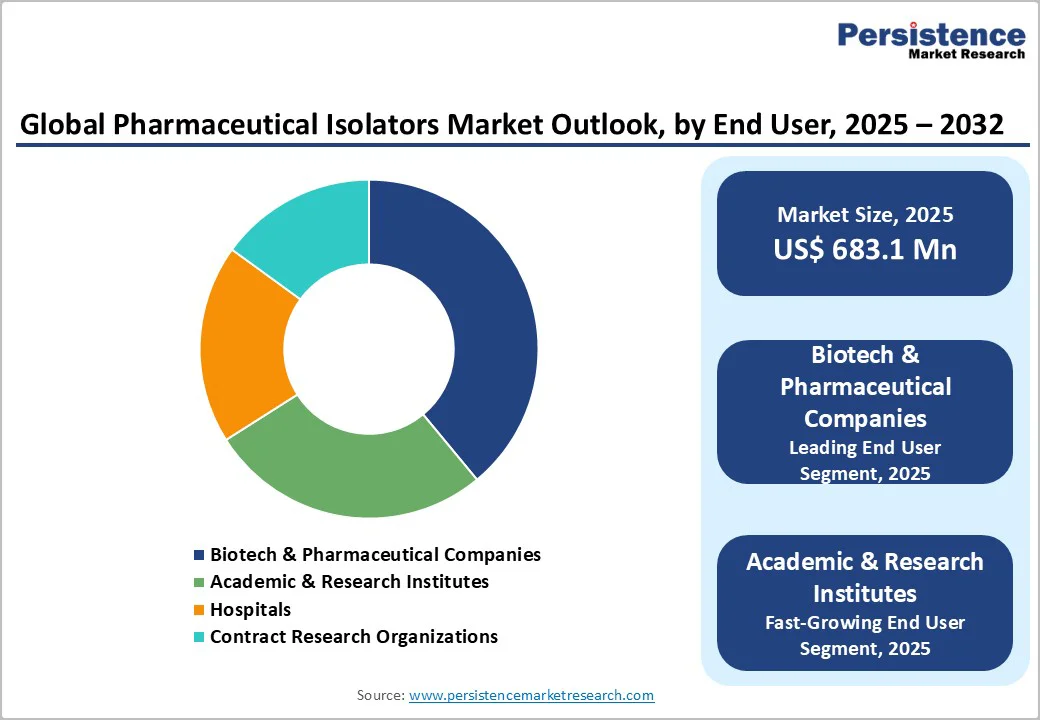
Biotech and pharmaceutical companies account for the largest share of the pharmaceutical isolators market due to their extensive involvement in aseptic manufacturing, biologics production, and handling high-potency drugs. These companies require isolators to maintain sterility, ensure regulatory compliance, and minimize contamination risks during critical processes such as fill-finish, sterility testing, and handling cytotoxic compounds. The growing demand for vaccines, gene therapies, and personalized medicines further drives isolator adoption in this segment. Additionally, large-scale operations and investments in advanced manufacturing facilities make biotech and pharma firms the primary end users, dominating the market over hospitals, research institutes, or contract organizations.
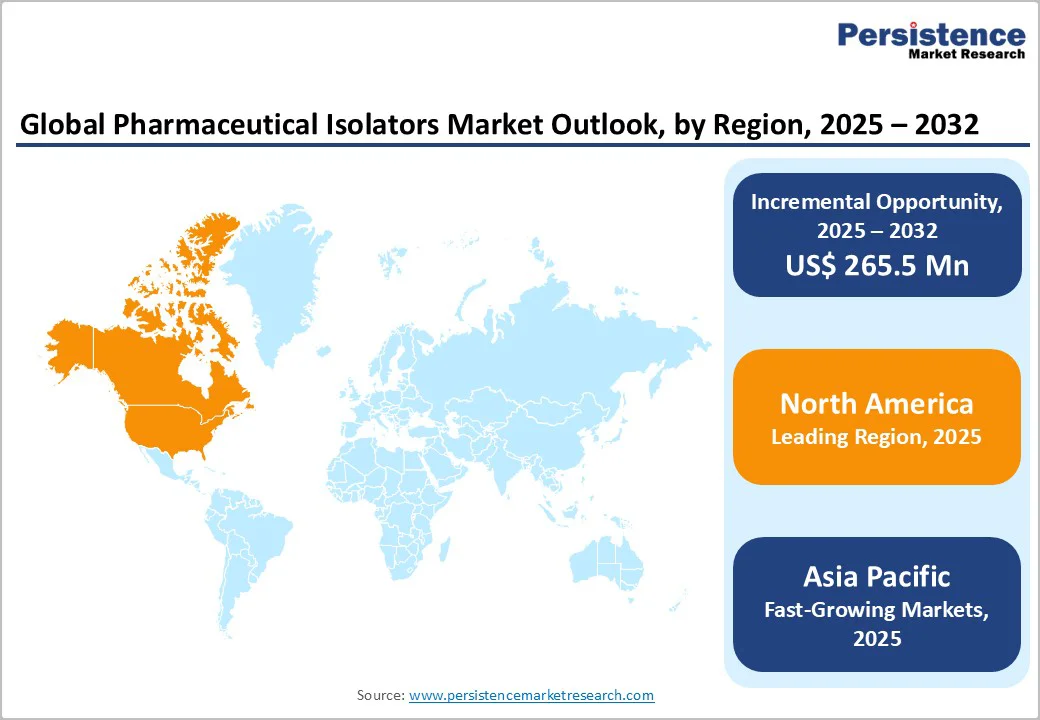
North America leads the pharmaceutical isolators market, driven by advanced pharmaceutical and biotechnology infrastructure, stringent regulatory standards, and high adoption of aseptic manufacturing technologies. The U.S., as the largest market within the region, contributes significantly due to its robust biologics, vaccine, and high-potency drug production capabilities. Strict FDA and USP guidelines encourage the use of closed isolators for sterility assurance, contamination control, and operator safety. Additionally, continuous investments in automated, modular, and IoT-enabled isolator systems support efficient and compliant operations. Rising demand for personalized therapies and the expansion of contract manufacturing organizations further reinforce North America’s dominant position in the global isolators market.
Asia Pacific pharmaceutical isolators market is emerging rapidly, fueled by expanding biopharmaceutical manufacturing, growing vaccine production, and increasing adoption of aseptic processing technologies. Countries like China, India, and South Korea are investing heavily in modern sterile facilities, driven by rising demand for biologics, generics, and high-potency drugs. Regulatory harmonization with global GMP standards and government incentives for pharmaceutical infrastructure are accelerating the adoption of isolators. Additionally, the presence of contract manufacturing organizations, increasing R&D activities, and the need for cost-effective, compact, and modular isolator systems are supporting market growth. This positions Asia Pacific as a high-potential region for isolator deployment.
The global pharmaceutical isolators market is highly competitive, dominated by global and regional players focusing on technological innovation, strategic partnerships, and customized solutions. Leading companies invest in advanced closed isolators, automation, and IoT-enabled systems to meet stringent regulatory requirements and growing demand for biologics, vaccines, and high-potency drugs.
The global pharmaceutical isolators market is projected to be valued at US$683.1 Mn in 2025.
The growing manufacturing of biologics, cell & gene therapies, and vaccines requires highly sterile environments, boosting isolator demand.
The global market is poised to witness a CAGR of 4.8% between 2025 and 2032.
Increasing oncology and high-potency API production drives demand for negative-pressure isolators.
Skan AG, Hosokawa Micron Ltd, Getinge AB, Azbil Telstar, and others
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn, Volume: Units |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By End-user
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author