ID: PMRREP32907| 199 Pages | 30 Dec 2025 | Format: PDF, Excel, PPT* | Healthcare

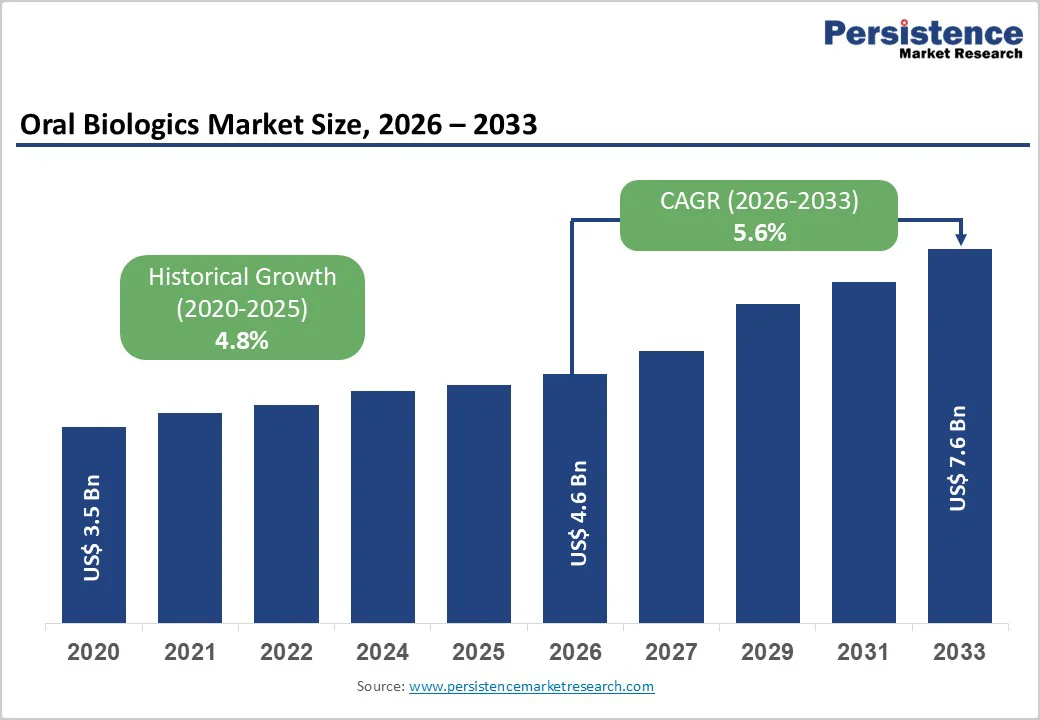
The global oral biologics market size is estimated to grow from US$ 4.6 Bn in 2026 to US$ 7.6 Bn by 2033. The market is projected to record a CAGR of 5.6% from 2026 to 2033.
Global demand for oral biologics is rising steadily, driven by the increasing prevalence of chronic autoimmune, inflammatory, and metabolic diseases requiring long-term therapeutic management. Conditions such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, and emerging cardiometabolic disorders are creating sustained demand for biologic therapies that improve efficacy while reducing treatment burden. Growing patient preference for noninjectable therapies, combined with improved medication adherence and convenience, is accelerating the shift toward oral biologic formulations. Advances in drug delivery technologies, including permeability enhancers, nanoparticle carriers, and molecular stabilization platforms, are enabling oral administration of complex biologics that were traditionally injectable. Rising survival rates and longer treatment durations among chronic disease patients further support market expansion. Increasing integration of biologics into precision medicine strategies, coupled with expanding access to specialty care and biologic reimbursement in both developed and emerging markets, continues to reinforce long-term global growth.
| Key Insights | Details |
|---|---|
| Oral Biologics Market Size (2026E) | US$ 4.6 Bn |
| Market Value Forecast (2033F) | US$ 7.6 Bn |
| Projected Growth (CAGR 2026 to 2033) | 5.6% |
| Historical Market Growth (CAGR 2020 to 2025) | 4.8% |
Driver – Rising Prevalence of Immune-Mediated Diseases and Advances in Oral Biologic Delivery Technologies
The rising global burden of chronic immunemediated and inflammatory disorders, including rheumatoid arthritis, psoriasis, inflammatory bowel disease, and cardiometabolic conditions, is a key driver of sustained growth in the oral biologics market. Increasing disease prevalence, earlier diagnosis, and improved survival rates are expanding the long-term treated patient population. Many of these conditions require lifelong therapy, driving consistent demand for effective and patient-friendly treatment options. As healthcare systems place greater emphasis on chronic disease management and long-term outcomes, biologic therapies continue to gain prominence across both developed and emerging markets.
Additionally, strong patient and physician preference for oral formulations is accelerating the adoption of oral biologics. Oral delivery significantly improves convenience, treatment adherence, and quality of life compared to injectable or infusion-based biologics, while also reducing dependence on hospital-based administration. Advances in oral drug delivery technologies—including permeability enhancers, enzyme inhibitors, nanoparticle carriers, and protective coating systems—are enabling stable and bioavailable oral administration of complex biologic molecules. Continuous innovation in formulation science is expanding the range of biologics that can be delivered orally, reinforcing the market’s longterm growth trajectory.
Restraints – High Development Costs and Limited Accessibility of Oral Biologic Therapies
Despite strong clinical potential, the oral biologics market faces significant restraints, including high development and manufacturing costs. Designing oral formulations for biologic molecules is technically complex, requiring advanced stabilization technologies, specialized production facilities, and extensive clinical validation. These factors contribute to higher product costs compared to conventional small-molecule oral drugs, limiting affordability in price-sensitive healthcare systems. In addition, lengthy regulatory approval processes and the need for long-term safety and efficacy data can delay commercialization timelines.
Limited reimbursement coverage for oral biologics in several regions further restricts patient access, particularly in low and middle-income countries. Variability in payer policies, budget constraints, and costcontainment measures often favors traditional injectable biologics or small-molecule alternatives. Moreover, uneven availability of specialists and advanced treatment centers in emerging markets can delay therapy initiation. Together, high pricing, reimbursement challenges, and healthcare infrastructure limitations constrain broader adoption, despite increasing clinical demand and growing physician awareness.
Opportunity – Expansion of Precision Medicine and Growing Acceptance of Oral Biologic Therapies
The rapid expansion of precision medicine is creating substantial growth opportunities for the global oral biologics market. The increasing use of biomarkers, genetic profiling, and targeted treatment strategies is driving demand for biologics personalized to individual patient needs. Oral biologics are particularly well-positioned within precision medicine frameworks, as they support long-term therapy adherence and outpatient-based care. Growing clinical evidence supporting efficacy and safety is encouraging wider acceptance of oral biologics across multiple lines of treatment.
Furthermore, healthcare systems worldwide are increasingly focused on reducing hospital visits, lowering administration costs, and improving patient-centric care models. This shift is favoring oral therapies that enable home-based treatment and long-term disease management. Rapid expansion of specialty care infrastructure in Asia Pacific, the Middle East, and Latin America, coupled with rising healthcare investments and pharmaceutical innovation, is expected to unlock new growth avenues. Strategic collaborations, pipeline expansion, and continued advancements in oral delivery technologies are likely to accelerate market expansion over the forecast period further.
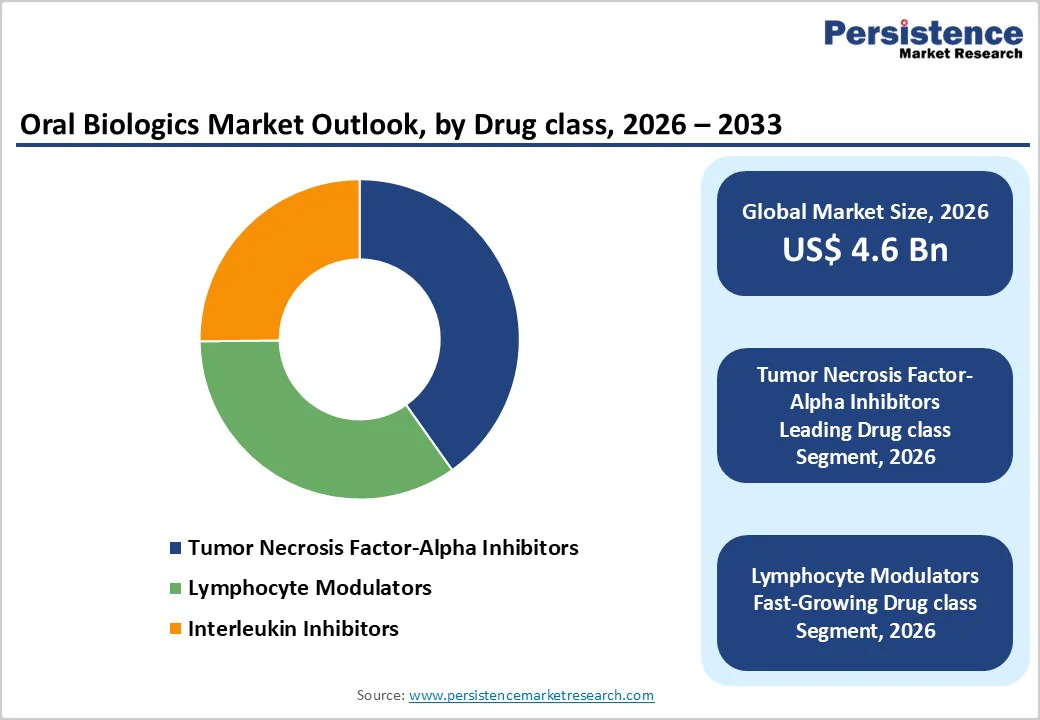
By Drug Class, Tumor Necrosis FactorAlpha Inhibitors Lead Due to Proven Clinical Outcomes and Broad Adoption
The tumor necrosis factor alpha inhibitors segment is projected to dominate the global oral biologics market in 2026, accounting for 40.2% of revenue. Segment leadership is supported by decades of clinical evidence, strong efficacy across multiple autoimmune diseases, and growing availability of oral formulations. TNFalpha inhibitors are widely prescribed as first-line or early-line therapies in conditions such as rheumatoid arthritis and inflammatory bowel disease, driving sustained demand. Physician familiarity, well-established treatment guidelines, and favorable payer coverage further reinforce adoption. While newer biologic classes are gaining traction, TNFalpha inhibitors continue to benefit from scale, accessibility, and strong long-term safety data.
By Indication, Rheumatoid Arthritis Remains the Largest Application Segment
The rheumatoid arthritis segment is expected to dominate the global oral biologics market in 2026, accounting for 28.1% of total revenue. This dominance is driven by the chronic nature of the disease, high prevalence, and long-term dependence on biologic therapies for disease control and symptom management. Oral biologics are increasingly preferred in rheumatoid arthritis due to improved patient compliance and reduced treatment burden compared to injectable options. Rising diagnosis rates, early treatment initiation, and expanding access to advanced therapies continue to support strong demand across both developed and emerging markets.
By Distribution Channel, Diagnostic Laboratories Hold the Largest Share
The diagnostic laboratories segment is projected to dominate the global oral biologics market in 2026, accounting for 45.0% of revenue. This leadership reflects the growing role of specialized laboratories in treatment monitoring, companion diagnostics, and therapeutic decision support associated with biologic therapies. High patient throughput, outsourcing trends from hospitals, and collaborations with pharmaceutical companies reinforce segment growth. Diagnostic laboratories also support longterm disease management and realworld evidence generation, strengthening their position within the oral biologics ecosystem.
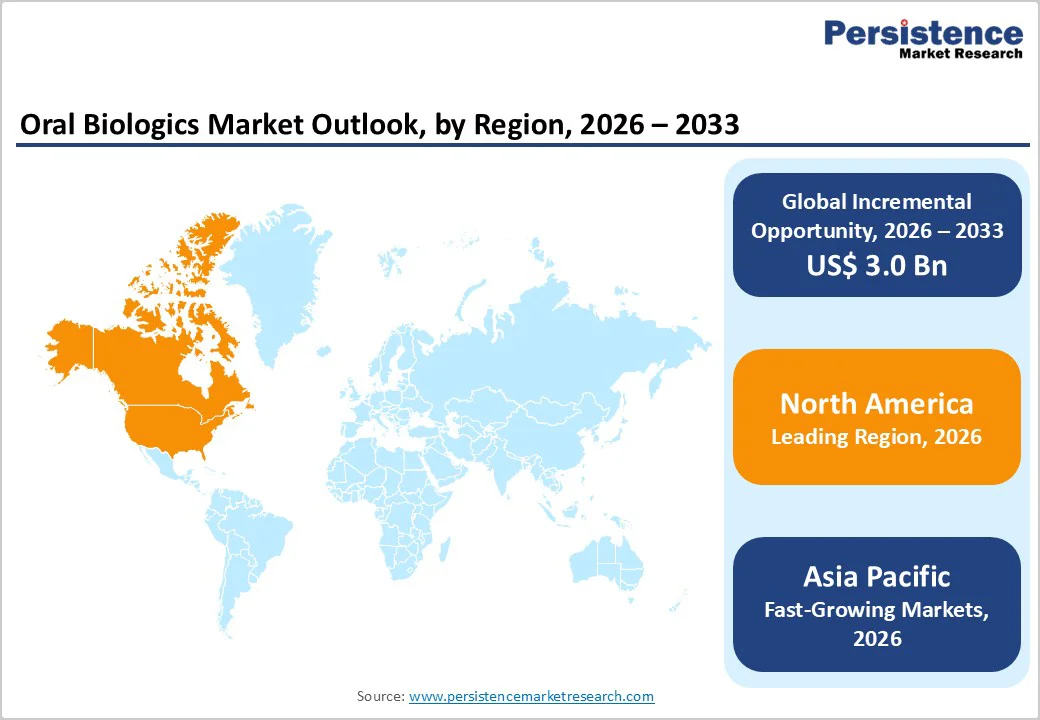
North America Oral Biologics Market Trends
The North American oral biologics market is expected to dominate globally with a value share of 48.3% in 2026, led primarily by the U.S. The region benefits from a highly advanced healthcare system, strong penetration of biologic therapies, and rapid adoption of innovative oral drug delivery technologies. High awareness among clinicians regarding autoimmune and inflammatory disorders supports early diagnosis and timely treatment initiation. Favorable reimbursement policies and broad insurance coverage significantly enhance patient access to high-cost biologic therapies, including oral formulations.
The presence of leading pharmaceutical companies, extensive clinical trial activity, and strong regulatory clarity further accelerates market adoption. In addition, North America has a robust research and innovation ecosystem, with continuous investment in next-generation biologics and immunemodulating therapies. Strong patient advocacy, well-established treatment guidelines, and early uptake of precision medicine approaches reinforce the region’s leadership in the global oral biologics market.
Europe Oral Biologics Market Trends
The Europe oral biologics market is expected to grow steadily, supported by a rising burden of chronic inflammatory diseases and increasing emphasis on longterm disease management. Countries such as Germany, the U.K., France, Italy, and the Nordic region are key contributors due to welldeveloped public healthcare systems and broad access to specialty medicines.
European healthcare frameworks prioritize evidence-based treatment and encourage the adoption of biologic therapies with demonstrated clinical value. The increasing use of oral biologics is supported by patient-centric care models aimed at improving adherence and reducing dependence on hospital-based treatment. Expansion of biologic reimbursement programs and growing real-world data supporting oral formulations are further strengthening market growth. Crossborder research collaborations, harmonized regulatory standards, and increasing investment in innovative drug delivery platforms continue to enhance Europe’s role in the global oral biologics landscape.
Asia Pacific Oral Biologics Market Trends
The Asia Pacific oral biologics market is expected to register a relatively higher CAGR of around 7.6% between 2026 and 2033, driven by a large patient population, rising disease awareness, and rapid expansion of healthcare infrastructure. Countries such as China, India, Japan, South Korea, and Southeast Asian nations are witnessing increasing diagnosis rates for autoimmune and inflammatory disorders, supporting biologics adoption.
Improving access to specialty care, rising healthcare expenditure, and government initiatives to strengthen chronic disease management are accelerating market growth. The expansion of hospital networks, specialty clinics, and trained healthcare professionals is improving the availability of advanced oral therapies. Additionally, collaborations between regional healthcare providers and global pharmaceutical companies are enhancing access to treatment. Growing emphasis on early intervention, long-term disease control, and patient-friendly treatment options is expected to sustain strong momentum for the adoption of oral biologics across the Asia Pacific region.
The global oral biologics market is highly competitive, with strong participation from Biocon Limited, Pfizer Inc., AbbVie Inc., Lumen Bioscience Inc., Novo Nordisk A/S, and Eli Lilly and Company. These companies benefit from robust biologic portfolios, advanced formulation capabilities, and strong global commercialization networks. Competitive strategies focus on expanding oral biologic pipelines, improving drug stability and bioavailability, and targeting additional immunemediated indications.
Investments in clinical trials, lifecycle management, and geographic expansion, particularly in emerging markets, are intensifying competition. Continuous innovation in oral delivery technologies and immune modulation is expected to drive sustained market evolution over the forecast period.
The global oral biologics market is projected to be valued at US$ 4.6 Bn in 2026.
The global oral biologics market is driven by rising prevalence of chronic autoimmune and inflammatory diseases and strong patient preference for oral, noninjectable biologic therapies that improve adherence and quality of life.
The global oral biologics market is poised to witness a CAGR of 5.6% between 2026 and 2033
Key opportunities in the global oral biologics market lie in the development of advanced oral delivery platforms for large molecules, expansion into cardiometabolic and gastrointestinal indications, and rapid uptake in emerging markets with improving access to specialty therapies.
Biocon Limited, Pfizer Inc., AbbVie Inc., Lumen Bioscience Inc., Novo Nordisk A/S, and Eli Lilly and Company are some of the key players in the oral biologics market.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Drug Class
By Indication
By Distribution Channel
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author