ID: PMRREP35631| 189 Pages | 19 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

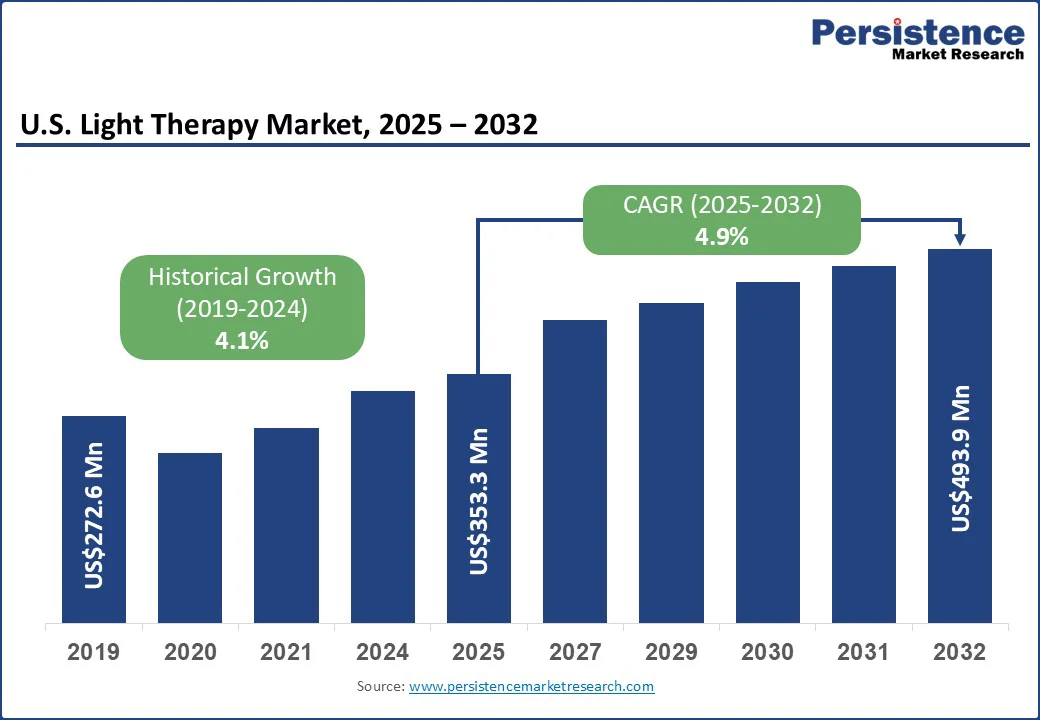
The U.S. light therapy market size is likely to be valued at US$353.3 Mn in 2025. It is expected to reach US$493.9 Mn by 2032, growing at a CAGR of 4.9% during the forecast period from 2025 to 2032, driven by rising awareness of mental health and sleep hygiene, increasing prevalence of lifestyle-related sleep disturbances, and a growing emphasis on non-pharmacological approaches to chronic conditions.
Key Industry Highlights:
| Key Insights | Details |
|---|---|
| U.S. Light Therapy Market Size (2025E) | US$353.3 Mn |
| Market Value Forecast (2032F) | US$493.9 Mn |
| Projected Growth (CAGR 2025 to 2032) | 4.9% |
| Historical Market Growth (CAGR 2019 to 2024) | 4.1% |
Seasonal Affective Disorder (SAD) and growing mental health awareness are key drivers of the U.S. light therapy market. Approximately 5% of U.S. adults experience full SAD annually, while another 10-15% report milder seasonal mood disruptions.
Beyond seasonal depression, about 13% of adolescents and adults experience depressive symptoms within any two weeks, highlighting a significant population that benefits from non-drug interventions.
Sleep disturbances, often linked to mood disorders, affect 14-15% of adults who have difficulty falling asleep and 17-18% who struggle to stay asleep. In contrast, nearly one-third of adults regularly get insufficient sleep.
These high prevalence rates, combined with public interest in safe, non-invasive treatments, are driving the adoption of light therapy solutions as effective tools for mood regulation and sleep improvement across the U.S. population. Growing awareness of mental health and wellness continues to reinforce the market’s long-term growth potential.
The lack of standardized treatment protocols in light therapy is a significant restraint in the U.S. market, particularly impacting the adoption and clinical acceptance of these therapies. For instance, while the World Association for Laser Therapy (WALT) has proposed dosage guidelines for various conditions, these recommendations vary widely.
For example, treatment durations range from 30 seconds to 10 minutes for 904-nm lasers, and from 20 seconds to 5 minutes for lasers in the 780-860 nm range, with energy levels varying from 5 to 500 mW. Such variability complicates the development of consistent treatment protocols.
Furthermore, the U.S. Food and Drug Administration (FDA) emphasized the need for clear clinical evidence to support at-home phototherapy claims, particularly for conditions such as acne and psoriasis. This regulatory scrutiny necessitates more rigorous clinical trials, which could delay product availability and increase costs, thereby affecting market growth.
Therefore, the absence of standardized treatment protocols and the need for robust clinical validation are pivotal challenges hindering the expansion of the light therapy market in the U.S.
The rising prevalence of skin disorders in the U.S. presents a significant opportunity for the expansion of light therapy treatments. According to the National Center for Complementary and Integrative Health, skin diseases affect as many as one in three Americans at any given time.
Conditions such as acne, psoriasis, and eczema are commonly treated with light therapy, including UVB phototherapy and intense pulsed light (IPL) systems, which have been FDA-cleared for these indications.
The lack of standardized treatment protocols remains a challenge. Variability in light wavelength, exposure time, and treatment parameters can lead to inconsistent outcomes. For instance, the MultiClearXL system is indicated for both UVB and visible blue/violet light treatments for conditions such as psoriasis and acne vulgaris.
Such variations underscore the need for standardized guidelines to ensure efficacy and safety. Addressing this gap through research and consensus could enhance the clinical adoption and effectiveness of light therapy in treating prevalent skin disorders.
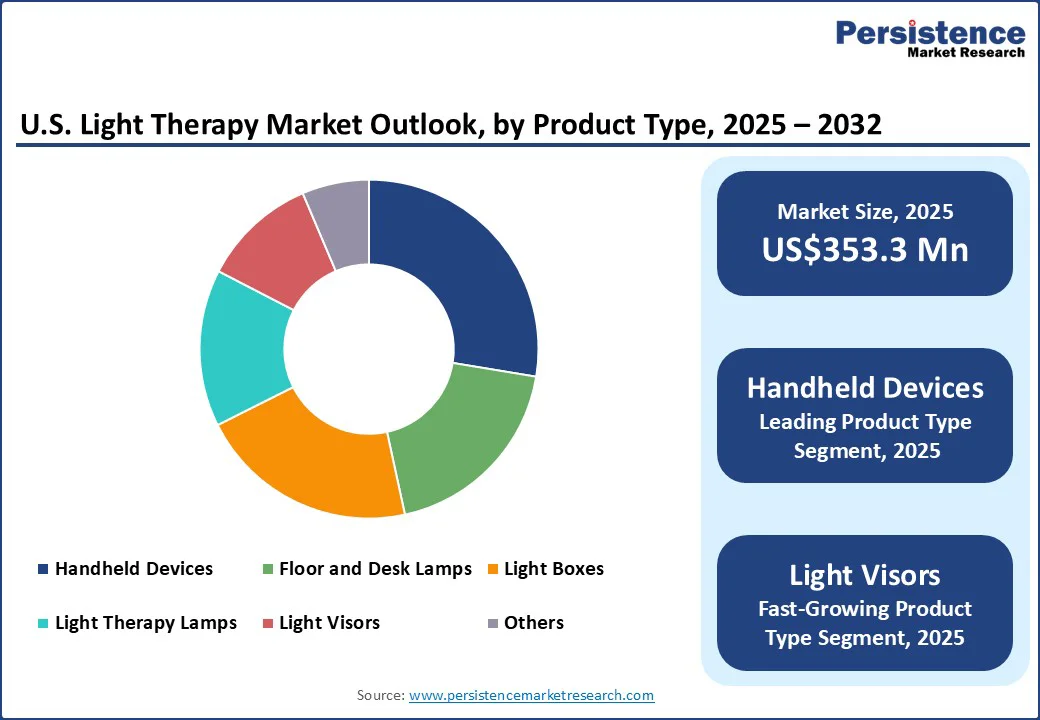
Handheld devices lead the U.S. light therapy market with a 27.6% share in 2025, due to their convenience, affordability, and versatility. Unlike large clinical units, these devices allow consumers to administer treatments at home, saving time and reducing dependency on professional clinics.
They are particularly popular among younger, tech-savvy users seeking non-invasive solutions for skin conditions such as acne, wrinkles, and hyperpigmentation, as well as for wellness purposes such as pain relief and mood enhancement.
The FDA has cleared several at-home handheld devices, boosting consumer confidence in their safety and efficacy. Moreover, their compact size and multi-mode functionality, offering red, blue, and infrared light in a single device, make them versatile for multiple applications. Increasing awareness of self-care and non-pharmaceutical interventions further drives their market dominance in the U.S. light therapy sector.
Skin conditions lead the U.S. light therapy market with a 34.7% share in 2025, as they represent the most common and visible health concerns that can be effectively managed with phototherapy. Conditions such as acne, psoriasis, eczema, and fine lines affect millions of Americans, creating consistent demand for safe and non-invasive treatments.
Light therapy, including blue, red, and near-infrared wavelengths, has been clinically validated to reduce inflammation, kill acne-causing bacteria, and promote collagen production, making it a preferred alternative to chemical-based treatments.
Additionally, FDA clearance of various devices for at-home use has made treatments more accessible, allowing users to address skin concerns conveniently. Rising consumer interest in aesthetics, anti-aging, and dermatological wellness, coupled with increasing awareness of the benefits of light therapy, further reinforces its dominance as the leading application segment in the U.S. market.
The U.S. light therapy market is competitive, led by players such as Joovv, Verilux, and HoMedics. These companies focus on device innovation, multi-wavelength functionality, portability, and FDA-cleared home-use solutions, while emerging players emphasize affordability, niche applications, and user-friendly designs to cater to skin, mental health, and wellness segments, targeting both clinical and at-home consumers.
The U.S. light therapy market is projected to be valued at US$353.3 Mn in 2025.
Rising skin disorders, mental health awareness, non-invasive treatments, technological advancements, and at-home device adoption drive growth.
The U.S. light therapy market is poised to witness a CAGR of 4.9% between 2025 and 2032.
Expanding mental health applications, rising skin disorders, technological innovations, at-home devices, and consumer wellness trends offer opportunities.
Major players in the U.S. light therapy market include Mito Red Light, Verilux, Infraredi, Celluma, Omnilux, and Joovv.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Mn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Application
By Light Type
By End-user
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author