ID: PMRREP35514| 184 Pages | 25 Jul 2025 | Format: PDF, Excel, PPT* | Healthcare

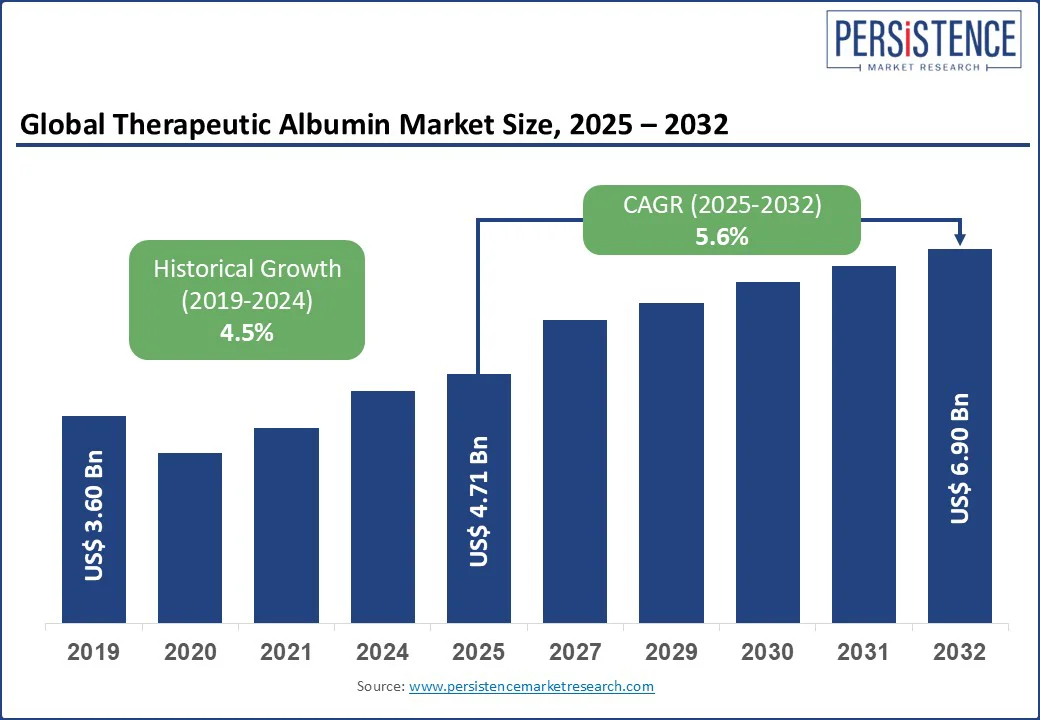
The global therapeutic albumin market size is likely to be valued at US$ 4.71 Bn in 2025 and is expected to reach US$ 6.90 Bn by 2032, growing at a CAGR of 5.6% during the forecast period from 2025 to 2032. The therapeutic albumin market is being driven by increasing demand for albumin-based therapies in critical care and chronic disease management. Therapeutic albumin, a vital plasma protein, plays a key role in treating conditions such as liver cirrhosis, nephrotic syndrome, and trauma-induced hypovolemia.
Advancements in biotechnology, rising awareness of albumin’s clinical benefits, and the expansion of healthcare infrastructure in emerging economies have driven market growth. Innovations in recombinant albumin production are expected to reduce reliance on human-derived sources, enhancing supply chain stability. As a result, the biopharmaceutical plasma protein market and clinical albumin therapy market are set to experience sustained momentum through 2032.
Key Industry Highlights:
| Market Attribute | Key Insights |
| Therapeutic Albumin Market Size (2025E) | US$ 4.71 Bn |
| Market Value Forecast (2032F) | US$ 6.90 Bn |
| Projected Growth (CAGR 2025 to 2032) | 5.6% |
| Historical Market Growth (CAGR 2019 to 2024) | 4.5% |
The increasing incidence of severe burn injuries and trauma cases globally is driving the demand for albumin-based fluid resuscitation therapies. Albumin’s ability to restore oncotic pressure and reduce edema makes it a preferred choice in emergency settings. According to the American Burn Association, over 450,000 patients in the U.S. receive treatment for burns annually. This surge in burn-related hospitalizations is fueling the growth of the clinical albumin infusion market, especially in advanced burn care units and trauma centers.
The therapeutic albumin market is witnessing a shift toward recombinant albumin manufacturing technologies, aimed at overcoming limitations of human-derived albumin. With over 10 million people affected by chronic liver and kidney diseases in the U.S. alone, the need for consistent and pathogen-free albumin products is rising. Recombinant variants offer improved scalability and safety, boosting adoption in the biopharmaceutical-grade albumin market. This innovation is particularly vital for long-term albumin therapy in nephrotic syndrome and cirrhosis patients.
The market faces a major bottleneck due to the restricted scalability of human plasma-derived albumin manufacturing. Albumin extraction depends heavily on voluntary blood donations, which vary across regions and seasons. This supply chain fragility leads to inconsistent availability and pricing. Moreover, the batch-to-batch variability in plasma-derived albumin formulations affects therapeutic consistency, especially in critical care settings. These limitations hinder the growth of the hospital-grade albumin therapy market, particularly in high-demand regions such as the Asia Pacific.
The recombinant therapeutic albumin market struggles with regulatory hurdles. Approvals for recombinant variants require extensive clinical validation to match the efficacy and safety of plasma-derived products. The high cost of recombinant albumin production technologies limits adoption in low- and middle-income countries. These challenges slow down the expansion of the biotechnology-based albumin formulation market, especially for chronic liver and kidney disease applications.
The development of albumin-based drug delivery platforms for targeted cancer therapy presents a major growth avenue. Albumin’s natural biocompatibility and ability to bind hydrophobic drugs make it ideal for delivering chemotherapeutics with enhanced bioavailability. This is accelerating innovation in the nanoparticle albumin-bound (nab) drug delivery market, especially for hard-to-treat cancers such as pancreatic and triple-negative breast cancer. Albumin is gaining traction in regenerative medicine scaffolding applications, where it supports cell adhesion and tissue regeneration.
The recombinant albumin for cell culture media market is expanding rapidly due to the growing demand for animal-free, consistent, and pathogen-free alternatives in biologics production. Recombinant albumin enhances cell viability and protein expression in vaccine and monoclonal antibody manufacturing. This trend is particularly strong in the Asia Pacific, where biopharma investments are surging. Moreover, the therapeutic-grade recombinant albumin market is benefiting from its integration into advanced therapies like gene editing and stem cell expansion.
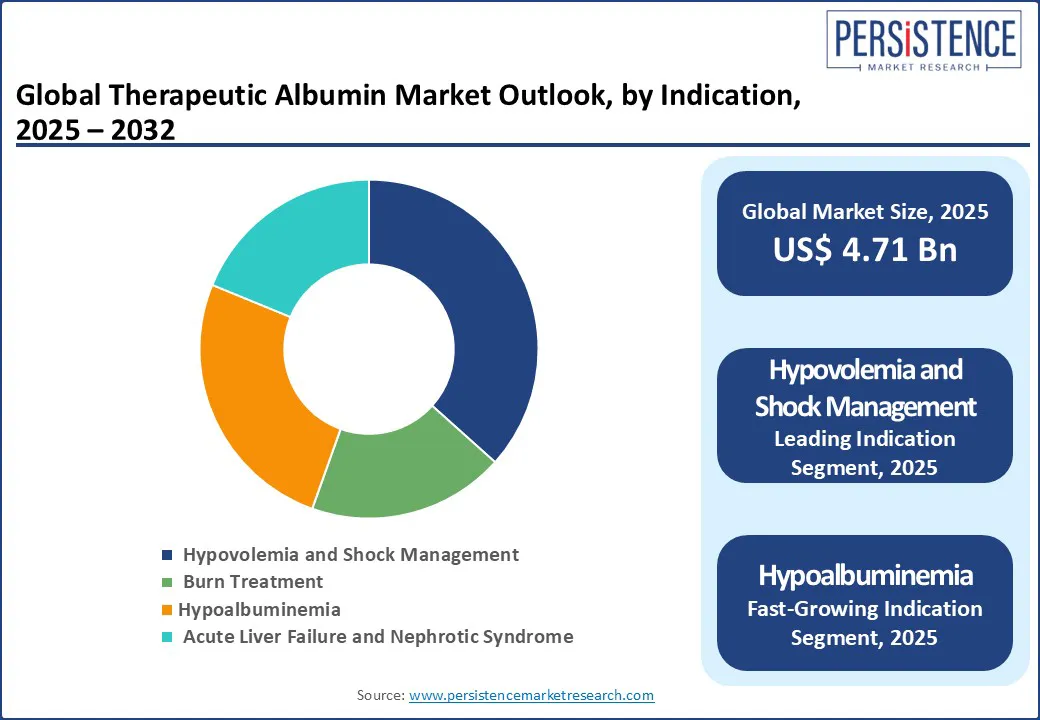
Among therapeutic indications, hypovolemia and shock management lead the albumin market, contributing around 37% of the total share. This dominance stems from the rising global incidence of trauma cases, road accidents, and surgical procedures that necessitate rapid fluid resuscitation. Albumin’s oncotic properties make it a vital plasma expander in managing blood volume loss, particularly in critical care units and emergency departments.
Hypoalbuminemia is the fastest-growing indication, fueled by the global rise in chronic liver disease, renal conditions, and an aging population. Advances in clinical nutrition and increased physician awareness are further reinforcing its uptake. Hypoalbuminemia, often associated with chronic liver diseases, nephrotic syndrome, and malnutrition, reflects the growing requirement for long-term albumin therapy. As the population ages and chronic diseases become more prevalent, demand for maintenance therapy is rising steadily. Burn treatment also constitutes a substantial segment, supported by high hospitalization rates and the critical role albumin plays in maintaining colloid osmotic pressure during extensive fluid shifts post-burn injuries. In many developing economies, improved access to burn care facilities is amplifying the demand for albumin.
Hospital pharmacies dominate the distribution landscape with a 65% market share, owing to the clinical nature of albumin use, particularly in ICU, perioperative, and trauma care settings. Hospitals also procure large volumes of albumin through tenders and institutional contracts, making them the largest end-user.
Online pharmacies are emerging as a convenient alternative, especially for recombinant and specialty albumin products. They are the fastest-growing distribution channel due to increased e-commerce, rising consumer preference for home-based care, and the shift toward digital-first healthcare models. Innovations in cold chain logistics and regulatory support for e-pharmacies are further accelerating growth.
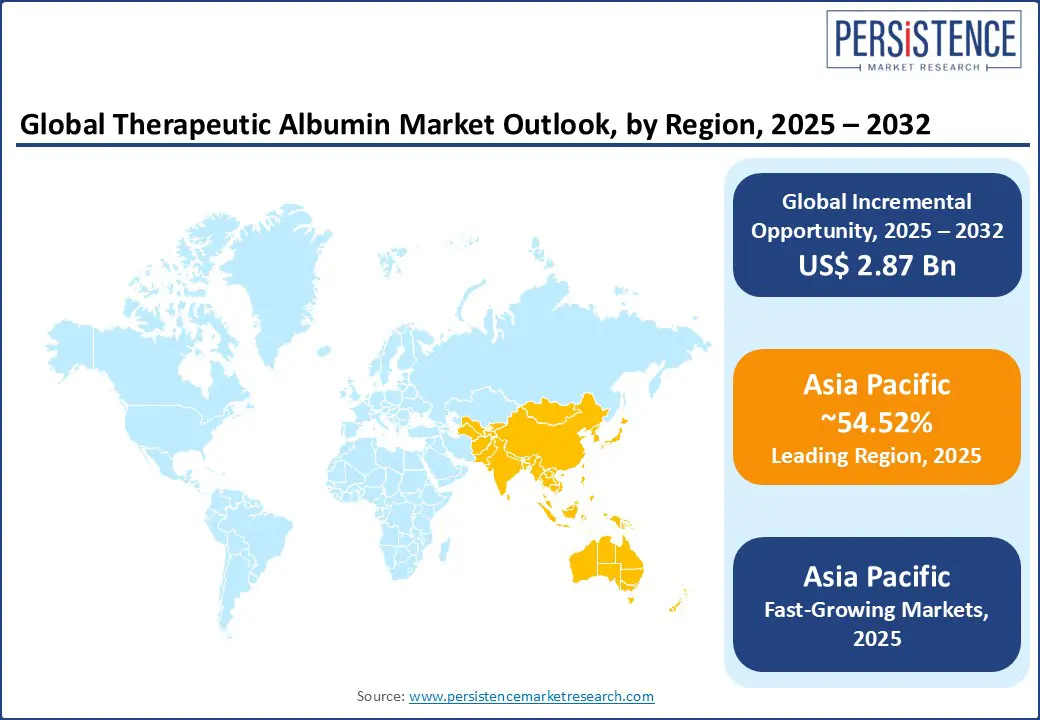
Asia Pacific is both the largest and the fastest-growing region in the therapeutic albumin market, accounting for a dominant 54.52% of the global share. This remarkable growth is driven by a large and aging patient population, the rising prevalence of chronic liver and kidney diseases, and significant improvements in healthcare infrastructure. Countries such as China, India, and Japan are at the forefront of this expansion. In China, government-backed initiatives under the 13th and 14th Five-Year Plans are accelerating biopharmaceutical development, particularly in plasma product manufacturing. The country also reports the world’s highest liver disease burden, making albumin a staple in cirrhosis and ascites management.
India is experiencing strong growth due to increasing cases of hypoalbuminemia linked to chronic kidney disease, malnutrition, and liver dysfunction. Nationwide programs such as Ayushman Bharat are improving access to albumin therapies, especially in public hospitals. Other regional players such as South Korea and Singapore are emerging as biotech hubs, focusing on vaccine manufacturing and cell culture applications where recombinant albumin is gaining traction. Furthermore, the demand for albumin in trauma and burn treatment remains high across Southeast Asia due to dense populations, frequent industrial accidents, and a growing focus on emergency preparedness.
North America holds the second-largest share of the therapeutic albumin market, supported by its well-developed healthcare infrastructure and consistent clinical demand. In the United States alone, over 6 million people live with kidney disease, and more than 4.5 million suffer from chronic liver conditions, both of which are major drivers of albumin-based therapies. The U.S. leads global innovation in recombinant albumin, with companies such as Grifols, CSL Behring, and Octapharma investing in next-generation plasma-derived biologics and specialty albumin formulations. Favorable regulatory support from the FDA and a proactive stance on biosimilars enhance innovation and fast-track approvals.
The U.S. is exploring albumin’s potential in targeted drug delivery, particularly in oncology, where it is used as a nanocarrier for chemotherapy agents. Canada also contributes to regional growth through its public healthcare system, which supports the use of albumin in nephrology and critical care, particularly for managing hypoalbuminemia and sepsis. The region benefits from high healthcare spending, a strong clinical research ecosystem, and extensive ICU infrastructure, ensuring stable and growing demand for therapeutic albumin.
Europe remains a key contributor to the global therapeutic albumin market, driven by well-established clinical guidelines, widespread adoption in surgical and intensive care settings, and a harmonized regulatory framework across the EU. Germany leads in albumin usage for transplant medicine, critical care, and surgical recovery. The country also shows growing adoption of recombinant albumin in biopharmaceutical processes such as cell therapy and biologic drug development.
The United Kingdom, through the National Health Service (NHS) and NIHR-backed studies, is exploring advanced clinical applications of albumin in liver failure and renal disorders. On the innovation front, Northern and Western European countries such as Sweden, Belgium, and the Netherlands are investing in biotech clusters that use recombinant albumin in cell culture, drug delivery, and vaccine production, adding a new layer of growth beyond traditional therapeutic indications.
The competitive landscape of global therapeutic albumin market is moderately consolidated, with high barriers to entry due to regulatory requirements, plasma sourcing challenges, and the need for advanced purification technologies.
A few dominant players, Grifols, S.A., Kedrion S.p.A., and Octapharma AG hold substantial market shares and lead in both product volume and innovation. The industry is also witnessing the emergence of smaller biotech firms and specialty manufacturers focused on recombinant albumin technologies. These entrants are introducing scalable, animal-free, and pathogen-free alternatives, especially for use in biopharmaceutical manufacturing, cell culture, and advanced therapies. Their presence is intensifying competition, particularly in niche and non-therapeutic segments.
The therapeutic albumin market is projected to reach US$ 4.71 Bn in 2025.
The therapeutic albumin market is expected to grow reach to US$ 7.55 Bn in 2032.
Key trends include the rise of recombinant albumin technologies, growing use in drug delivery and regenerative medicine, and increasing demand in critical care and chronic disease management.
Hypovolemia and shock management is the leading segment, contributing 37% of the market share.
The therapeutic albumin market is expected to grow at a CAGR of 7.0% from 2025 to 2032, driven by rising demand in emergency and chronic care.
Major players include Grifols, S.A., Kedrion S.p.A., Octapharma AG, CSL Behring, Takeda Pharmaceutical Company Limited.
| Report Attribute | Details |
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis Units | Value: US$ Bn/Mn, Volume: As Applicable |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
| Customization and Pricing | Available upon request |
By Source
By Indication
By Distribution Channel
By Application
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author