ID: PMRREP35679| 199 Pages | 6 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

The global mRNA therapeutics market size is expected to reach US$6.0 billion by 2025. It is estimated to reach US$21.4 billion by 2032, growing at a CAGR of 20.0% during the forecast period from 2025 to 2032, driven by the expanding adoption of messenger RNA (mRNA) technology beyond infectious diseases into oncology and rare genetic disorders.
This growth is driven by advances in lipid nanoparticle (LNP) delivery, AI-enabled mRNA design, and increasing R&D investment, which boosts therapeutic precision and efficacy. Regulatory backing and the expansion of clinical pipelines further accelerate the adoption of personalized medicine.
| Key Insights | Details |
|---|---|
|
mRNA Therapeutics Market Size (2025E) |
US$6.0 Bn |
|
Market Value Forecast (2032F) |
US$21.4 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
20.0% |
|
Historical Market Growth (CAGR 2019 to 2024) |
12.0% |
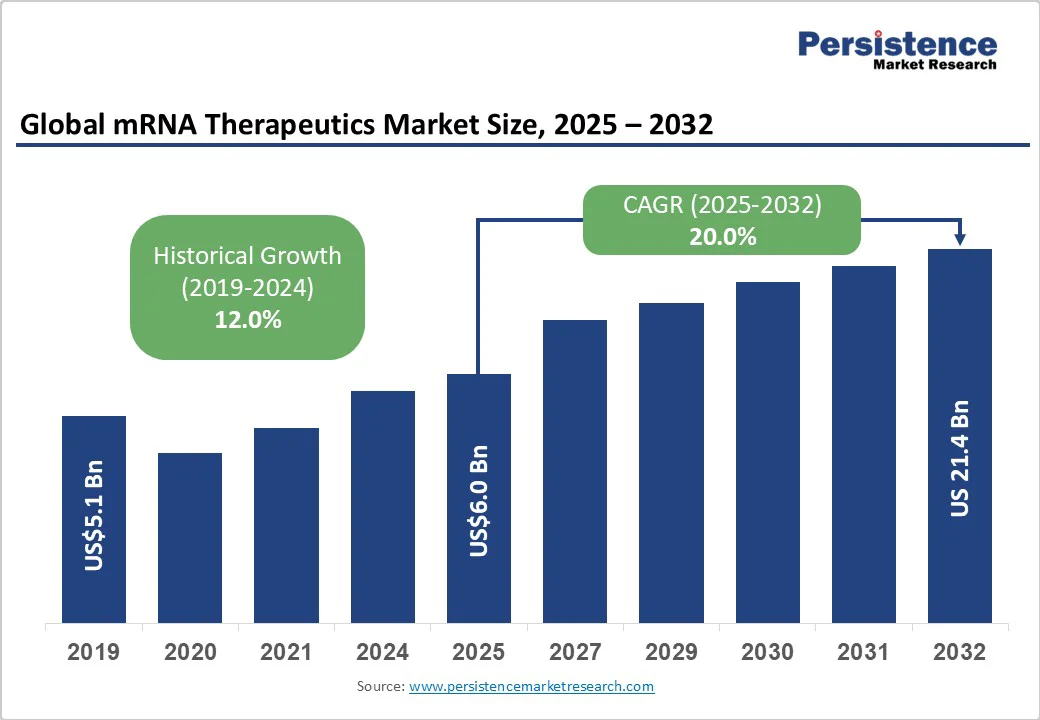
The success of mRNA COVID-19 vaccines has validated the platform, driving its expansion into oncology and rare genetic disorders, where disease prevalence continues to rise. According to the WHO, 2022 saw 20 million new cancer cases and nearly 10 million related deaths. Advances in gene editing and protein replacement have further broadened mRNA’s utility, while industry leaders now invest over US$5 billion annually in R&D to mature pipelines. Regulatory bodies, such as the FDA and EMA, are evolving approval pathways to accommodate these innovations, thereby enabling faster patient access.
Cutting-edge delivery systems, particularly lipid nanoparticles (LNPs), have significantly enhanced mRNA stability and cellular targeting, which are critical to therapeutic success. Technological advancements have reduced immunogenicity and degradation, thereby enhancing safety. Simultaneously, AI is revolutionizing mRNA sequence design by predicting stability and immune responses, thereby cutting development timelines by up to 30%. Manufacturing breakthroughs, including scalable microfluidics and automation, are reducing production costs by over 15% annually. Regulatory pilot programs now support AI-driven drug development, further accelerating the commercialization of these innovations. These developments are lowering barriers to entry, enabling personalized medicine, and transforming mRNA into a multi-therapeutic platform with diversified revenue streams, attracting strong venture capital and public investment.
The manufacturing and distribution of mRNA therapeutics face significant cost and logistical hurdles. Production costs for mRNA vaccines currently range from $ 150 to $ 200 per dose, which is vastly higher than the $ 3 to $ 5 cost for conventional vaccines. This disparity is largely due to complex synthesis processes and the requirement for ultra-cold storage (as low as -70°C). These cold chain demands increase distribution costs by up to 25% compared to traditional biologics and pose challenges in low- and middle-income countries lacking robust infrastructure. These cost burdens limit pricing flexibility, hinder market penetration, and contribute to supply inconsistencies that disrupt both clinical trials and commercial rollouts. Strategic investments in cold chain technology and scalable manufacturing are crucial for reducing costs and enhancing global access.
Regulatory hurdles continue to complicate the landscape of mRNA therapeutics. Despite progress in evolving frameworks, inconsistent global standards and the requirement for extensive long-term safety data remain significant barriers to approval. mRNA drugs, especially those targeting rare diseases, often face slow clinical recruitment and protracted development cycles, typically spanning 7 to 10 years, as estimated by the FDA. These challenges heighten development costs and risks, potentially deterring investor interest. Harmonizing international regulatory standards and expanding accelerated approval pathways are crucial to overcoming these obstacles and promoting the broader adoption of mRNA-based therapies.
The integration of AI into mRNA therapeutic development offers transformative prospects. AI-driven molecular modeling enables the creation of personalized mRNA sequences tailored to individual genetic profiles, advancing precision medicine applications for oncology and rare diseases. Machine learning algorithms enhance clinical trial design, optimizing patient selection and monitoring, thereby reducing time-to-market by up to 25.0%. AI-powered mRNA drug discovery platforms are attracting investments of millions from the technology and pharmaceutical sectors. Capitalizing on these synergistic technologies enables faster innovation cycles, improved trial success rates, and optimized resource allocation, positioning firms for competitive advantage in an increasingly complex market environment.
Another opportunity for mRNA therapeutics market players lies in the emerging economies of the Asia Pacific and Latin America, where annual healthcare expenditure is growing at a steady rate, healthcare infrastructure is improving rapidly, and government and private companies are investing in biotech innovation hubs and manufacturing capacities. Governments are enacting supportive policies, including expedited approval processes for innovative therapies and financial incentives for biotech startups. For example, China anticipates tripling its mRNA manufacturing output capacity by 2030.
Furthermore, these regions are experiencing an unprecedented rise in chronic disease burden, necessitating widening of access to new therapies for patients. Strategic partnerships between multinational firms and local players are accelerating technology transfer and market penetration, presenting actionable avenues for investors and companies aiming to capture untapped demand and cost-efficient manufacturing benefits.
The therapeutic mRNA vaccines segment is currently the largest, set to account for an estimated 55.0% share in 2025. This segment has established a strong market foundation due to the rapid deployment of COVID-19 vaccines and the continuation of vaccination programs for other infectious diseases. The sustained interest in prophylactic vaccines further fortifies this segment's position. The large manufacturing infrastructure and accelerated regulatory approvals for vaccines compared to therapeutics contribute to its leadership.
The therapeutic mRNA protein segment is emerging as the fastest-growing segment, forecasted to expand at a CAGR of approximately 21% from 2025 to 2032. Growth in this segment is primarily driven by advancements in personalized medicine and increasing applications in oncology, rare genetic disorders, and protein replacement therapies. This segment's growth reflects the evolution of therapeutic pipelines and an increasing trend toward treating complex diseases with mRNA platforms, offering lucrative opportunities for companies to diversify and innovate.
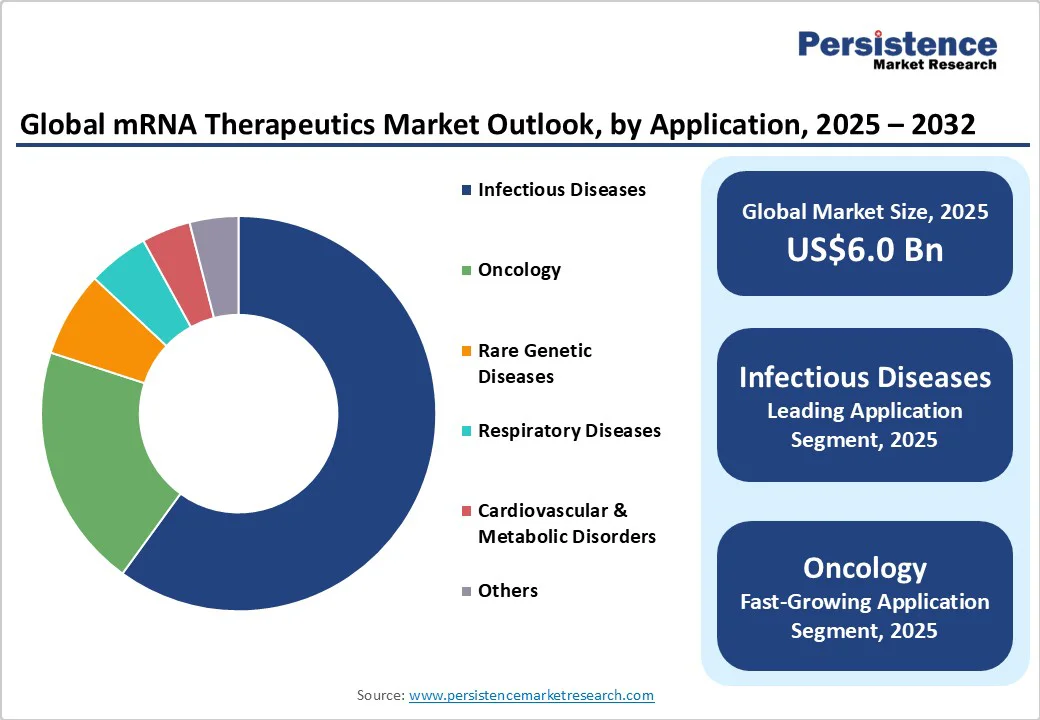
Infectious diseases remain the dominant application area for mRNA therapeutics in 2025, accounting for a 60% revenue share. This dominance is driven by the ongoing need for vaccines against COVID-19 variants and preventative vaccines targeting other viral infections, such as respiratory syncytial virus (RSV) and influenza. Clinical efficacy and public health initiatives underpin sustained demand in this segment.
Conversely, oncology represents the fastest-growing application. Tumor-targeting mRNA vaccines and immunotherapies are rapidly advancing through clinical trials, driven by the growing global incidence of cancer and substantial investments in cancer research. The oncology segment is experiencing significant expansion due to the push toward personalized immunotherapy solutions, which necessitate highly adaptable mRNA platforms.
Lipid nanoparticles (LNPs) are the prevailing delivery system for mRNA therapeutics, commanding over 70.0% market share in 2025. The predominance of LNPs is attributed to their ability to protect mRNA molecules from degradation and enhance cellular uptake, which has been crucial to the success of mRNA COVID-19 vaccines. Regulatory approvals to date have favored the LNP platform, reinforcing its dominance.
Polymeric nanoparticles and other emerging delivery systems are the fastest evolving, with a projected CAGR of approximately 18.0% through 2032. These alternative delivery technologies offer potential advantages, including lower immunogenicity and improved targeting capabilities. Their development is driven by the need to optimize therapeutic outcomes and reduce side effects, especially for chronic and personalized treatments. Continued innovation in this segment is expected to challenge LNP dominance and widen the therapeutic applicability of mRNA drugs.
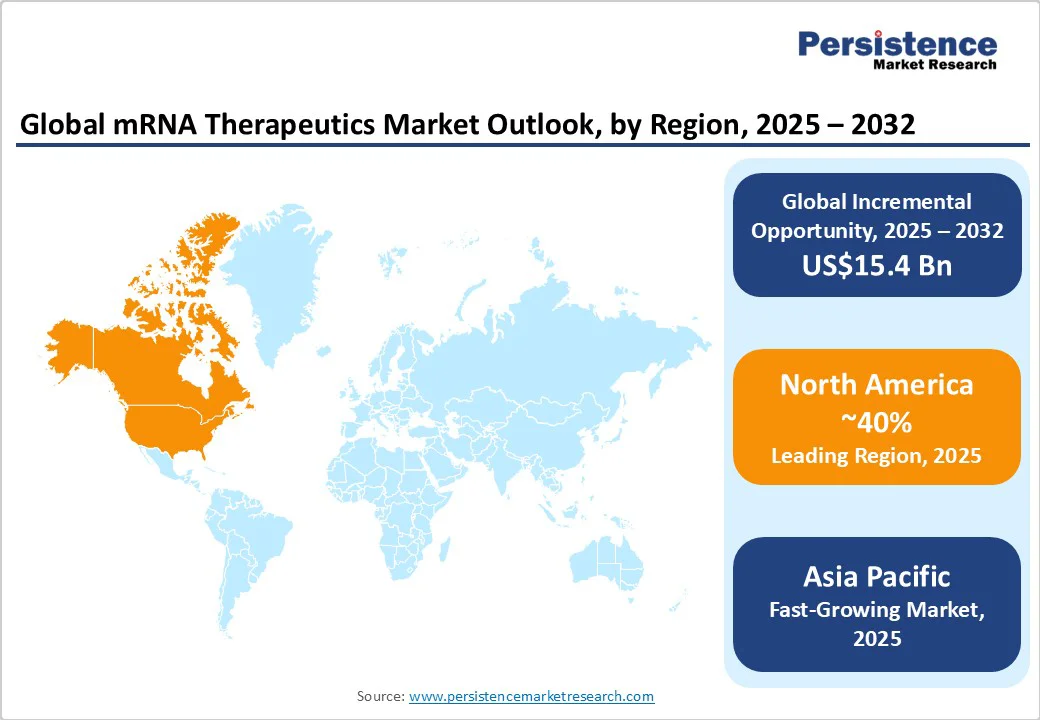
North America maintains its leadership position in the mRNA therapeutics market share, capturing approximately 40.0% in 2025. The regional market, led by the United States, benefits from a well-established healthcare ecosystem, substantial government and private funding exceeding US$10 Billion annually, and a progressive regulatory environment facilitated by the FDA. The COVID-19 pandemic accelerated mRNA technology adoption, establishing a mature innovation ecosystem with a concentration of leading players such as Moderna, Pfizer, and BioNTech.
The North America market is projected to expand at a CAGR of 14.0% from 2025 to 2032, backed by ongoing expansion in oncology clinical programs and personalized medicine development. Robust cold chain infrastructure and investment in biomanufacturing scale-ups underpin consistent supply capabilities. Additionally, collaborative initiatives between academia, industry, and government agencies are accelerating therapeutic breakthroughs, offering sustained growth and investment opportunities in the region.
Europe is expected to hold an estimated 25.0% market share in 2025, with Germany, the U.K., France, and Spain being key contributors. The market for mRNA therapeutics here is expected to grow at a CAGR of approximately 12.0% through 2032. The growth prospects of the regional market are bolstered by a supportive regulatory framework harmonized under the EMA, which facilitates cross-border clinical trials and market access. Government investments and funding programs are nurturing a vibrant biotech sector, leading to innovative pipeline developments.
Europe's established pharmaceutical manufacturing base, combined with stringent pharmacovigilance standards, ensures product quality and patient safety. Strategic alliances between European and multinational companies have fostered knowledge transfer and accelerated commercialization. Growing patient populations affected by cancer and infectious diseases, coupled with increasing awareness of precision medicine, augment demand. The evolving regulatory and competitive landscape renders Europe a significant and strategic market for mRNA therapeutics innovation and commercialization.
Asia Pacific is forecasted to be the fastest-growing regional market for mRNA therapeutics with a projected CAGR exceeding 18.0% through 2032. Key markets, including China, Japan, India, and countries within ASEAN, are driving market growth through expanding healthcare infrastructure and modernization of regulatory pathways, enabling faster clinical approvals. Large patient populations and escalating chronic disease prevalence stimulate demand for innovative therapeutics. Local governments are promoting biotech innovation through financial incentives, infrastructure development, and public-private partnerships.
Additionally, technology transfer agreements and collaborations with global pharmaceutical entities are enhancing regional capabilities in mRNA manufacturing, projected to substantially reduce costs and improve supply chain resilience. Asia Pacific is strategically poised to become a global hub for manufacturing and consumption of mRNA therapeutics, offering vast growth and investment potential enabled by favorable demographic and economic trends.
The global mRNA therapeutics market is moderately concentrated, with key players, Moderna, Pfizer, BioNTech, and CureVac, which comprise around 64.0% of the market share. These firms leverage strong pipelines, advanced manufacturing capabilities, and global distribution to dominate the vaccine segment. Emerging biotech companies are gaining footholds in therapeutic mRNA applications and innovating novel delivery systems. Market concentration is rising due to mergers and strategic partnerships, though fragmentation remains in early-stage therapies and regional markets, offering entry points for new players.
Leading companies are focused on innovation, expanding into personalized treatments and rare diseases. They pursue cost efficiency via manufacturing scale-ups and strategic expansion into high-growth emerging markets. Increasingly, partnerships with AI and digital health firms are driving faster discovery and development, shaping the next phase of competitive differentiation.
The mRNA therapeutics market is projected to reach US$6.0 Billion in 2025.
The expanding use of mRNA technology beyond infectious diseases, particularly in oncology and rare genetic disorders, alongside breakthroughs in delivery platforms such as lipid nanoparticles, is driving market growth.
The mRNA therapeutics market is poised to witness a CAGR of 20.0% from 2025 to 2032.
Refinements in mRNA sequence design enabled by AI and machine learning, strong regulatory support, and increased commercialization of personalized medicine are key market opportunities.
Moderna, Inc., Pfizer Inc., and BioNTech SE are some of the key players in the mRNA therapeutics market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Application
By Delivery System
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author