ID: PMRREP35454| 175 Pages | 27 Jun 2025 | Format: PDF, Excel, PPT* | Healthcare

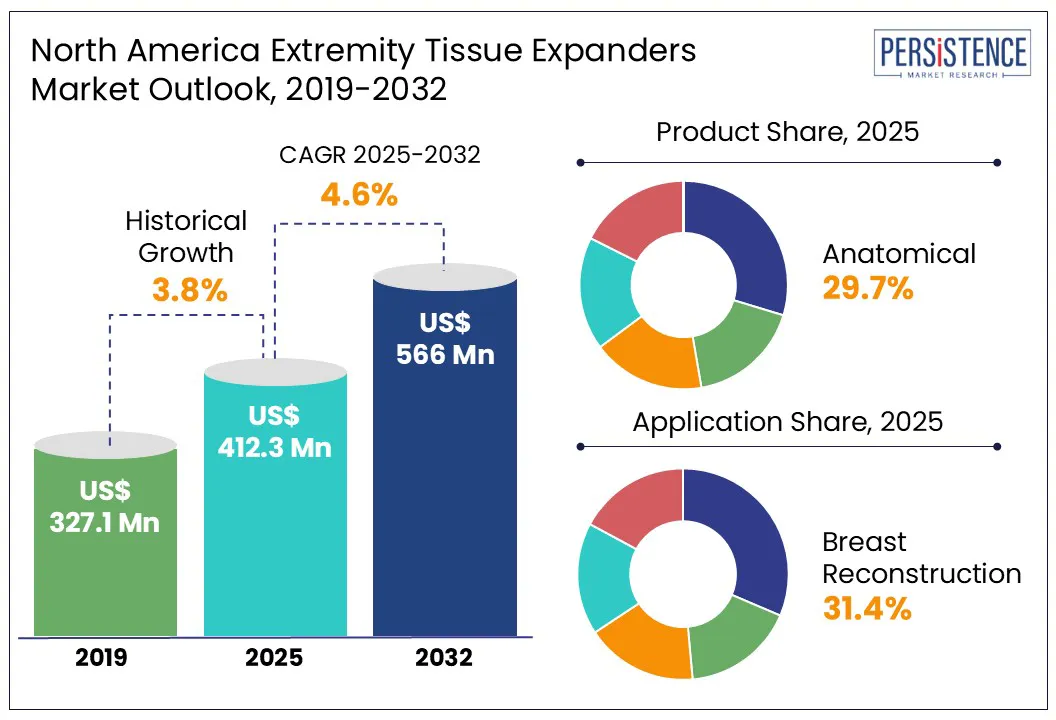
The North America extremity tissue expanders market size is likely to be valued at US$ 412.3 Mn in 2025 and is expected to reach US$ 566.0 Mn by 2032, growing at a CAGR of 4.6% in the forecast period between 2025 and 2032, reveals a Persistence Market Research report.
The North America extremity tissue expanders market is gaining traction due to reconstructive surgical requirements in trauma care, oncology, and congenital defect correction. Once a niche product associated with breast reconstruction, tissue expanders are now finding significant application in preparing complex anatomical sites for definitive surgical repair. With the rise in cases of road traffic injuries and limb-threatening infections, the use of expanders has shifted from elective cosmetic procedures to essential steps in limb salvage and functional recovery.
Key Industry Highlights
|
Market Attribute |
Key Insights |
|
North America Extremity Tissue Expanders Market Size (2025E) |
US$412.3 Mn |
|
Market Value Forecast (2032F) |
US$566.0 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
4.6% |
|
Historical Market Growth (CAGR 2019 to 2024) |
3.8% |
Increasing incidence of breast cancer is expected to bolster the North America extremity tissue expanders market growth. According to the American Cancer Society, in the U.S., nearly 316,950 new cases of invasive breast cancer will be diagnosed in women in 2025. A significant proportion of diagnosed women are now opting for mastectomy followed by reconstruction. This surge has led to a consistent increase in two-stage breast reconstruction procedures involving tissue expanders. Hence, manufacturers have intensified their research and development activities and extended their distribution networks.
Growth in breast cancer cases has further increased surgeon familiarity with expander-based procedures, including expansion timelines, infection management, and volume control. This procedural expertise is increasingly being applied to limb, head, and neck reconstructions. Additionally, the volume-driven economies of scale achieved through breast cancer-related demand have made tissue expanders more accessible for other specialties. Bulk procurement, streamlined regulatory pathways, and accelerated innovation cycles have allowed companies to repackage or adapt their breast tissue expander technology into extremity-specific models.
The risk of complications such as infection, hematoma, seroma, and tissue necrosis continues to limit the widespread adoption of extremity tissue expanders in North America. In procedures involving the lower extremities, poor perfusion and limited soft tissue coverage significantly increase the risk of necrosis or infection following expander placement. A 2022 multicenter online study reported complication rates of up to 38% in lower limb expansion cases, with skin necrosis and delayed wound healing being the most frequent issues.
Seroma formation is also a prominent concern. In extremity applications where mobility cannot be fully restricted, constant motion can lead to mechanical irritation around the expander. In outpatient orthopedic settings, this has led some clinicians to prefer immediate flap coverage or grafting rather than relying on staged expansion protocols. It is particularly evident in aging patients or those with diabetes, who present high vascular compromise.
Trauma-related injuries and severe road accidents are significantly pushing the clinical scope for extremity tissue expanders in North America. As per the Centers for Disease Control and Prevention (CDC), the U.S. witnesses over 2 Mn emergency department visits annually owing to motor vehicle crashes. Many of these involve compound fractures, extensive soft tissue loss, and open wounds in the upper or lower extremities. In such cases, tissue expanders are increasingly being used to gradually regenerate skin and soft tissue coverage before definitive reconstruction with bone grafts or prosthetics.
A notable shift is occurring in Level I trauma centers, where orthopedic and plastic surgeons are collaborating on limb-preserving procedures rather than defaulting to amputation. Tissue expansion plays a key role in these multi-stage reconstructions, mainly for injuries around joints or areas with insufficient donor tissue. The opioid crisis has further contributed to a rise in high-energy traumas, including crush injuries sustained from machinery, falls, or traffic accidents involving intoxicated individuals. These injuries often result in soft tissue loss around the tibia, femur, or forearm, zones where expanders are now being deployed to improve skin availability.
Based on product, the North America extremity tissue expanders market is segregated into anatomical, round, rectangular, and crescent. Out of these, anatomical extremity tissue expanders are anticipated to hold around 29.7% share in 2025 due to their ability to conform precisely to complex and irregular anatomical sites such as curved limb surfaces. These expanders are pre-shaped to mimic the natural curvature and shape of limbs, reducing the risk of implant migration, asymmetrical expansion, or skin distortion. In surgeries involving elbows, knees, ankles, and pediatric extremities, where space is limited, anatomical expanders offer superior functional and cosmetic outcomes.
Round extremity tissue expanders, on the other hand, are predicted to witness a considerable growth rate through 2032 with their operational simplicity, predictable expansion patterns, and compatibility with a wide range of reconstructive procedures. Their uniform radial expansion allows surgeons to manage soft tissue regeneration without the complexity of anatomical shaping. This makes them suitable for standardized applications such as bone graft site preparation, post-tumor excision reconstruction, and compartment syndrome recovery. Another significant advantage of round expanders is their adaptability across both the upper and lower extremity procedures.
By application, the market is divided into breast reconstruction, scalp reconstruction, oral and maxillofacial reconstruction, and pediatrics. Among these, breast reconstruction will likely account for approximately 31.4% of the North America extremity tissue expanders market share in 2025 amid the high volume of post-mastectomy procedures and the established use of expanders as a standard step in two-stage breast reconstruction. As per Plastic and Reconstructive Surgery, in the U.S. alone, more than 100,000 breast reconstruction surgeries are performed every year. A substantial portion of these involve tissue expanders to gradually prepare the chest wall for permanent implants.
Oral and maxillofacial reconstruction is predicted to showcase a significant CAGR from 2025 to 2032 due to the rising number of complex facial trauma cases, tumor resections, and congenital craniofacial abnormalities that require staged reconstruction. While not involving limbs, maxillofacial regions are considered extremity-like in terms of surgical planning complexity, anatomical irregularity, and tissue coverage demands. Expanders are used in these procedures to generate soft tissue volume before bone grafting, flap transfers, or implant placement. Pediatric craniofacial surgery also utilizes tissue expanders, especially for conditions such as hemifacial microsomia, Treacher Collins syndrome, or giant congenital nevi.
In the U.S., extremity tissue expanders represent a mature and steadily expanding niche in reconstructive surgery. The market is primarily spurred by rising demand in trauma reconstruction, congenital deformity correction, and burn treatment. The country shows high procedural integration, with expanders used in about 61% of reconstructive surgeries involving extremities such as arms, hands, legs, and feet. Adoption is particularly strong in upper extremity reconstruction, including hand and arm surgeries.
Key players in the U.S. are investing in product differentiation through novel materials and design innovations. A significant development came in October 2023, when Establishment Labs received FDA 510(k) clearance for its Motiva Flora SmoothSilk expander featuring MRI-conditional, non-magnetic ports. In addition, there is a surging preference among U.S. surgeons for anatomically contoured expanders, which offer better fit and performance in complex joint areas, including knees and elbows.
In Canada, the market is currently witnessing steady growth, supported by the country’s robust trauma care infrastructure and increasing demand for reconstructive procedures. Increasing cases of traumatic injuries from sports-related incidents and road accidents, which frequently require limb reconstruction, are predicted to drive the market. Canada’s universal healthcare system also facilitates patient access to such specialized procedures, mainly in urban trauma and burn centers.
Clinical adoption is specifically high in pediatric and lower limb reconstruction. Congenital limb deformities, often treated early in childhood, have increased the use of tissue expanders made for small anatomical sites. Complication rates in these pediatric cases, however, can be high owing to tissue fragility and compliance issues during long expansion timelines. Surgical teams continue to accelerate procedural techniques and postoperative care, helping eliminate these risks and enhance outcomes.
The North America extremity tissue expanders market consists of a few specialized medical device manufacturers, each striving to address the unmet demands of reconstructive and orthopedic surgeries. Leading players are competing through product customization, rapid post-surgical expansion technologies, and compatibility with complex anatomical sites. A handful of local players are investing in anatomically contoured expanders and self-inflating hydrogels to reduce patient discomfort and minimize the requirement for frequent clinical interventions.
The North America extremity tissue expanders market is projected to reach US$ 412.3 Mn in 2025.
Favorable reimbursement policies and the rising patient awareness about limb-preserving options are the key market drivers.
The North America extremity tissue expanders market is poised to witness a CAGR of 4.6% from 2025 to 2032.
Introduction of AI-based surgical planning tools and development of hybrid expanders for use in bone interface zones are the key market opportunities.
Mentor Worldwide LLC, Sientra, Inc., and PMT Corporation are a few key market players.
|
Report Attribute |
Details |
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis Units |
Value: US$ Bn/Mn, Volume: As Applicable |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
|
Customization and Pricing |
Available upon request |
By Product
By Extremity
By Application
By End-use
By Country
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author