ID: PMRREP30058| 149 Pages | 29 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

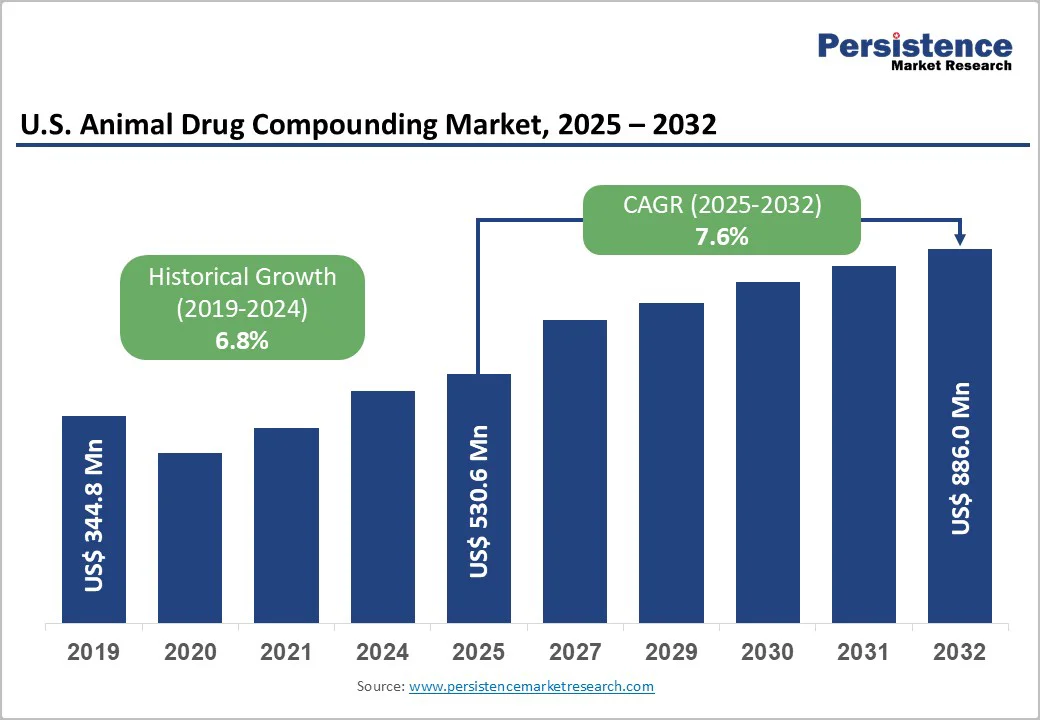
The U.S. animal drug compounding market size is valued at US$530.6 million in 2025 and projected to reach US$886.0 million at a CAGR of 7.6% during the forecast period from 2025 to 2032. The U.S. animal drug compounding market is growing rapidly, driven by increasing demand for customized veterinary medications.
The rising prevalence of pet and livestock diseases, the focus on preventive care, and the adoption of personalized treatments are fueling growth. Innovations in compounding techniques, such as flavored formulations and precise dosing, enhance treatment effectiveness, supporting market expansion in companion animals and livestock healthcare.
| Key Insights | Details |
|---|---|
|
U.S. Animal Drug Compounding Market Size (2025E) |
US$ 530.6 Mn |
|
Market Value Forecast (2032F) |
US$ 886.0 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
7.6% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.8% |
The growing demand for tailored and customized medications to address the unique health needs of pets is a major driver for the U.S. animal-drug compounding market. In the United States, nearly 94 million households, representing about 66 % of all homes, now own at least one pet. Of these, approximately 68 million households have a dog, and 49 million households have a cat. Because conventional veterinary medications often come in standard strengths or forms that may not suit every species, age group or breed, the demand for compounded solutions, tailored dosages, flavors, or novel delivery modes has risen. Customized formulations allow veterinarians to adjust for weight, species-specific metabolism, or unique conditions, supporting better compliance and outcomes. As pet ownership climbs and client expectations for personalized care increase, the U.S. animal-drug compounding market is positioned for significant expansion.
The absence of standardized federal guidelines in the U.S. for compounding animal drugs has created a restraint for the animal drug compounding market. According to the Food and Drug Administration (FDA), animal drugs compounded from bulk drug substances are not FDA-approved, meaning they have not been reviewed for safety, effectiveness or quality manufacturing. Moreover, the United States Government Accountability Office (GAO) reported that the FDA “does not currently have final guidance directing its regulatory approach on drug compounding for animals” and lacks complete documentation of its enforcement actions.
Because of this regulatory gap, there is risk of misuse or overuse of compounded products. For example, errors in potency (either too much or too little active ingredient) have caused animal deaths. These uncertainties in regulation and quality reduce veterinarian/caregiver confidence and pose a barrier to the full maturation of the U.S. animal drug-compounding market.
The adoption of digital prescription management represents a notable opportunity for the U.S. animal-drug compounding market. As veterinary practices increasingly digitize workflows, around 60% of vet clinics in North America now use electronic health records, and over 80% of independent vet pharmacies report contracting with digital systems, yet many still rely on fax-and-phone methods for prescriptions.
For example, a new digital tool launched by Wag! enables veterinary clinics to e-prescribe medications, reducing manual burden and improving medication accuracy. These digital platforms help streamline the prescribing of compounded drugs, which is important where dosage, formulation and species difference matter thus improving efficiency, safety and compliance. With pets in over two-thirds of U.S. households and more complex therapeutic needs, leveraging digital prescription management can reduce errors, cut administrative time, and expand access to compounded treatments, supporting growth of the U.S. animal-drug compounding market.
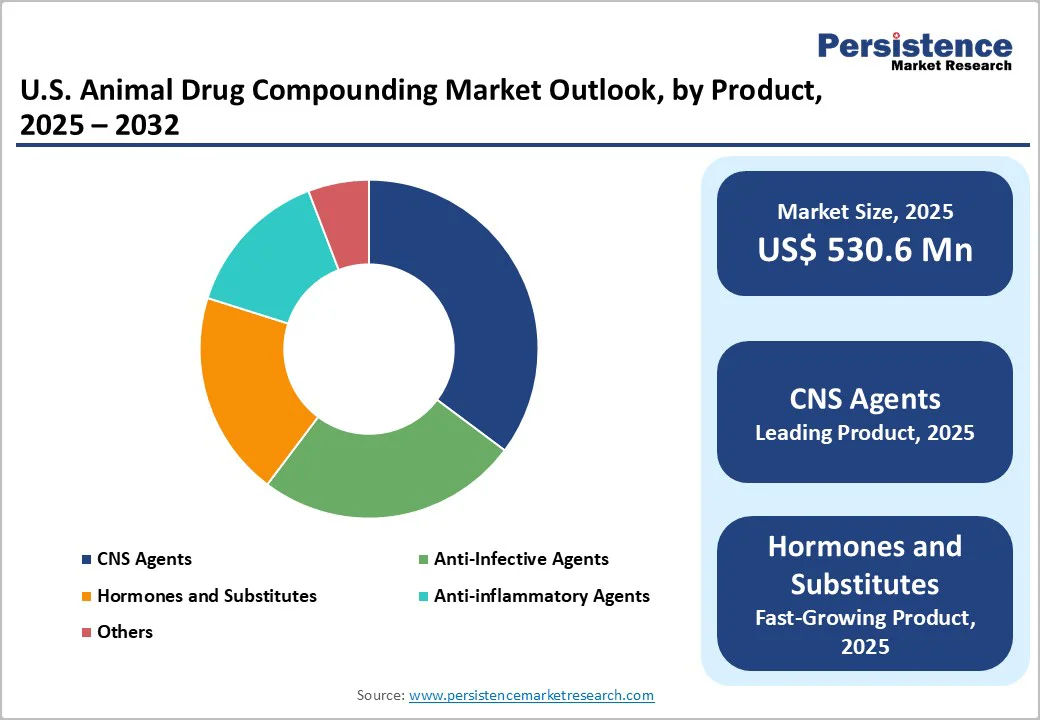
CNS Agents lead the product category in the market with 35.2% share in 2025 due to high prevalence of neurological and behavioral issues in companion animals. For example, a U.S. study of over 43,000 dogs found that 99.1% displayed at least one moderate-to-severe behavioral issue such as anxiety, fear or separation attachment. Because many FDA-approved veterinary drugs don’t come in formulations or doses tailored for diverse species, breeds, ages or sizes, veterinarians frequently rely on compounded CNS medications to address seizures, anxiety and behavioral disorders. According to the Food & Drug Administration (FDA), compounded animal drugs are often used when no approved drug exists for a species or condition, even though they are not FDA-approved and thus carry a greater risk. Given this combination of high clinical need and tailored dosing requirements, CNS agents emerge as the leading product category in the U.S. animal-drug compounding space.Top of Form
Bottom of Form
The dominance of oral formulations in the U.S. animal drug compounding market is driven by their practicality, ease of administration, and versatility for companion animals and livestock. Many pets, particularly cats and dogs, resist swallowing pills, making flavored liquids, suspensions, or chewable forms essential for compliance. Oral compounded medications allow precise dosing tailored to an animal’s weight, species, and condition, which is critical for chronic treatments such as pain management, hormonal therapies, and CNS disorders.
Additionally, veterinary practitioners often need to adjust drug concentrations because commercially available drugs may not be suitable for all sizes or breeds. Studies indicate that oral medications are overwhelmingly preferred in clinical practice due to their non-invasive nature and ability to maintain long-term compliance. This combination of ease, customization, and patient acceptability makes oral formulations the dominant choice in the U.S. animal drug compounding market.
The competitive landscape for the U.S. animal drug-compounding market is marked by a mix of specialized veterinary compounding pharmacies and larger multi-service companies. These players focus on tailored formulations, flavor customization, and niche animal-species solutions. Regulatory compliance and quality accreditation (such as by the Food and Drug Administration) serve as key differentiators. Larger firms are increasingly scaling production, automating operations, and forming partnerships with veterinary clinics to extend reach.
The U.S. animal drug compounding market is projected to be valued at US$ 530.6 Mn in 2025.
Rising pet ownership, increasing disease prevalence, demand for personalized medications, and technological advancements drive the U.S. animal drug compounding market.
The U.S. animal drug compounding market is poised to witness a CAGR of 7.6% between 2025 and 2032.
Opportunities include developing novel formulations, expanding veterinary clinic services, adopting digital prescriptions, AI-driven dosing, and targeting underserved animal healthcare segments.
Hoye's Pharmacy, Vertisis Custom Pharmacy, Dougherty's Pharmacy, Triangle Compounding Pharmacy Inc., Medisca Inc., Wedgewood Pharmacy.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Animal
By Formulation
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author