ID: PMRREP32912| 289 Pages | 2 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

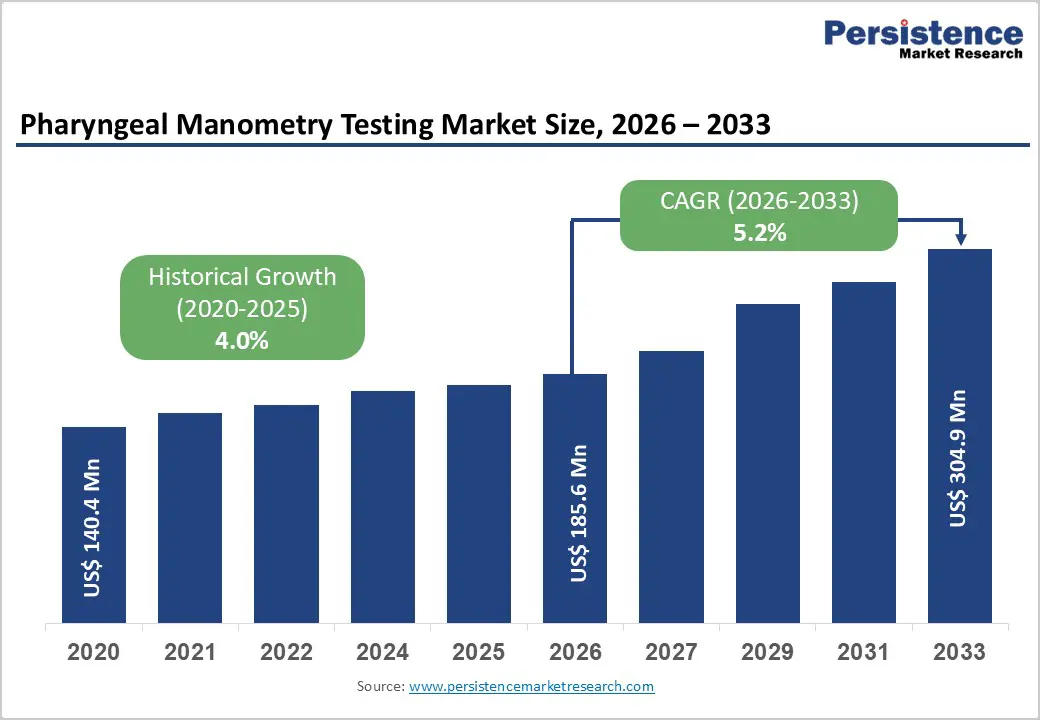
The global pharyngeal manometry testing market size is estimated to grow from US$ 232.6 Mn in 2026 to US$ 382.1 Mn by 2033. The market is projected to record a CAGR of 5.2% during the forecast period from 2026 to 2033.
Global demand for pharyngeal manometry testing is rising steadily, driven by the increasing prevalence of inherited ocular disorders, including inherited retinal diseases (IRDs), congenital cataracts, corneal dystrophies, optic neuropathies, and syndromic eye conditions linked to systemic genetic aMnormalities. The growing burden of rare genetic eye diseases, combined with improving survival rates and longer life expectancy among pediatric and adult patients, is increasing long-term diagnostic, prognostic, and monitoring requirements. Expanding newborn and pediatric screening initiatives, improved access to molecular diagnostics, and rising indicate awareness among ophthalmologists and genetic specialists are contributing to sustained testing volumes. Rapid advances in genomic technologies—particularly next-generation sequencing (NGS), whole-exome sequencing (WES), and whole-genome sequencing (WGS)—are enhancing diagnostic accuracy, reducing turnaround times, and enabling earlier disease detection. Increasing integration of genetic testing into precision ophthalmology workflows, alongside rising healthcare investments in specialized diagnostic laboratories and tertiary eye-care centers, is accelerating global adoption. Concurrently, ongoing research into novel gene–phenotype correlations and disease pathways continues to reinforce long-term market expansion across both developed and emerging regions.
| Key Insights | Details |
|---|---|
|
Pharyngeal Manometry Testing Market Size (2026E) |
US$ 232.6 Mn |
|
Market Value Forecast (2033F) |
US$ 382.1 Mn |
|
Projected Growth (CAGR 2026 to 2033) |
5.2% |
|
Historical Market Growth (CAGR 2020 to 2025) |
4.0% |
Driver – Rising Prevalence of Mental Health Disorders and Advancements in Psychedelic Therapeutics Driving Market Growth
The rising global prevalence of mental health disorders, including treatment-resistant depression, post-traumatic stress disorder (PTSD), anxiety disorders, and substance use disorders, is a primary driver fueling sustained demand for psychedelic drugs. Increasing societal awareness, reduced stigma, and recognition of the long-term economic and social burden of mental illness are contributing to broader acceptance of novel therapies. Traditional pharmacological and psychotherapy approaches often show limited efficacy in complex or chronic cases, creating a significant unmet need that psychedelic-assisted therapies are increasingly positioned to address.
Technological and clinical advancements are significantly accelerating market growth. Progress in formulation development, controlled dosing systems, and therapy-assisted delivery protocols is improving safety, consistency, and patient outcomes. Clinical studies demonstrating robust efficacy in both adult and adolescent populations are enhancing physician confidence and regulatory acceptance. Integration of digital health platforms, neuroimaging, and biomarker-based patient monitoring further enhances precision treatment approaches, enabling tailored therapy regimens. Collectively, increasing prevalence of mental health conditions, growing acceptance of psychedelics as a legitimate treatment option, and continuous innovation in delivery and monitoring technologies are driving steady and long-term expansion of the global psychedelic drugs market.
Restraints – High Treatment Costs and Limited Access to Specialized Therapy Limiting Market Adoption
High costs associated with psychedelic-assisted therapies remain a key restraint, particularly in low- and middle-income regions. Expenses related to clinical administration, therapist-led sessions, facility infrastructure, patient monitoring, and ongoing follow-up care can limit patient access and adoption. While research is expanding, the intensive supervision and multidisciplinary support required for safe and effective treatment increase overall costs. Additionally, insurance coverage and reimbursement frameworks for psychedelic therapies are still in early stages, restricting widespread uptake and creating financial barriers for patients.
Limited availability of trained therapists, psychiatrists, and multidisciplinary clinical teams further constrains market penetration. Shortages of qualified personnel capable of delivering therapy-assisted treatments limit the number of treatment centers, particularly outside urban or research-focused regions. Regulatory hurdles, variations in treatment protocols, and a lack of standardized guidelines across jurisdictions can also impede adoption. In many emerging markets, fragmented mental health infrastructure and limited awareness of therapy benefits contribute to delayed or missed treatment opportunities. These economic and structural barriers continue to restrain broader market growth despite increasing recognition of clinical efficacy and demand for psychedelic drugs.
Opportunity – Expansion of Clinical Trials and Precision Psychiatry Creating New Growth Opportunities
The rapid expansion of clinical research programs and investigator-led studies represents a significant growth opportunity for the global psychedelic drugs market. Governments, academic institutions, and biotechnology companies are increasingly funding trials to explore psychedelic therapies for depression, PTSD, anxiety, and addiction. This expansion enables robust evidence generation, regulatory engagement, and eventual market authorization, while creating confidence among healthcare providers and patients. Early intervention programs, particularly in at-risk populations, are emerging as strategic pathways for therapy adoption.
Furthermore, the growing field of precision psychiatry is creating new demand across both clinical and research settings. Patient stratification using biomarkers, neuroimaging, and behavioral profiling allows therapies to be tailored for maximum efficacy, enhancing treatment outcomes and safety. Integration of digital therapeutics, telehealth monitoring, and outcome-tracking platforms is enabling scalable deployment in both urban and semi-urban centers. Investments in specialized treatment centers, training programs for certified therapists, and collaborative research initiatives are strengthening market foundations. As precision psychiatry continues to evolve, demand for personalized psychedelic therapies is expected to expand substantially, supporting long-term growth and establishing new avenues for innovation.
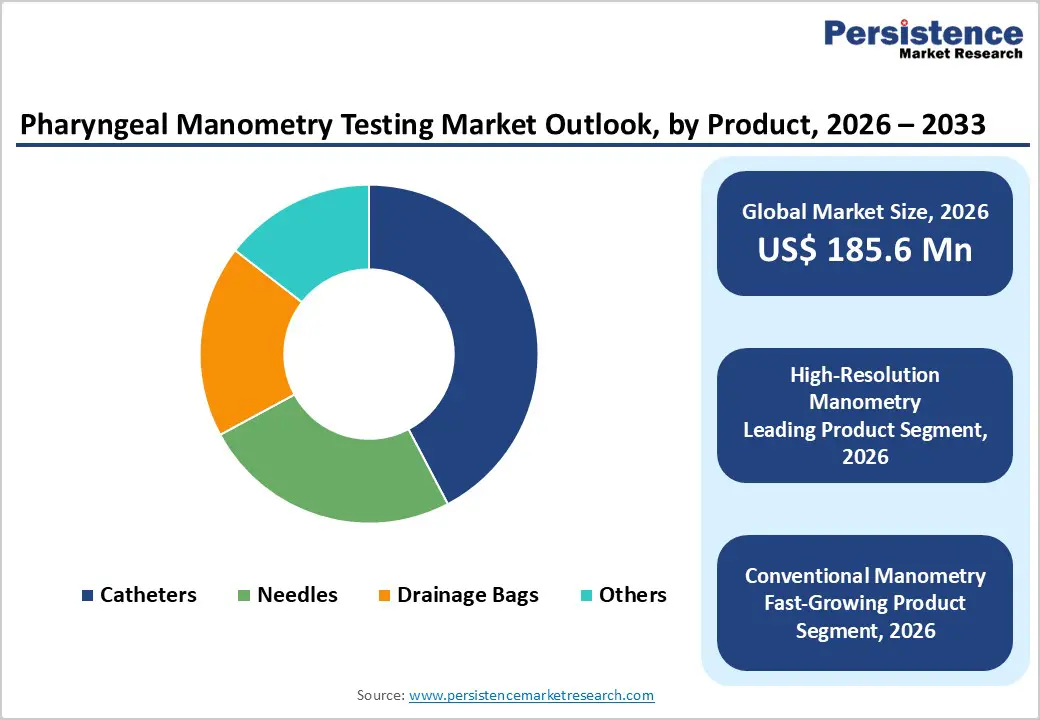
By Product, High-Resolution Manometry Owing to Comprehensive Coverage and Cost Efficiency
The high-resolution manometry segment is projected to dominate the global psychedelic drugs market in 2026, accounting for a revenue share of 60.0%. Its leadership is driven by the growing clinical preference for advanced, high-precision delivery and monitoring technologies that support safe and controlled administration of psychedelic therapies. High-resolution systems enable detailed physiological and neurological assessment during treatment, supporting improved patient stratification, dosing accuracy, and outcome monitoring. These platforms offer an optimal balance between clinical effectiveness, operational efficiency, and scalability, making them suitable for use in specialized mental health clinics and hospital-based programs. Physicians and clinical researchers increasingly favor such advanced systems as first-line tools in psychedelic-assisted therapy protocols due to their ability to generate reliable, actionable data without excessive procedural complexity. Established clinical trial protocols, rising practitioner familiarity, and compatibility with digital health and neuro-monitoring platforms further support adoption. While complementary technologies continue to emerge, high-resolution solutions remain central to psychedelic therapy workflows due to their reliability and clinical practicality.
By Application, Gastroesophageal Reflux Disease (GERD) Emerges as the Dominant Segment Driven by Clinical Necessity
The gastroesophageal reflux disease (GERD) segment is projected to dominate the global psychedelic drugs market in 2026, accounting for a revenue share of 45.5%. Segment leadership is attributed to the high prevalence of chronic, treatment-resistant mental health conditions that require novel therapeutic approaches, including anxiety disorders, depression, and stress-related comorbidities frequently observed alongside GERD. Psychedelic-assisted therapies are increasingly being explored for their potential to address underlying psychological contributors to chronic gastrointestinal symptoms, driving research and clinical interest. Growing evidence supporting mind–gut interactions and neuropsychiatric modulation is reinforcing the relevance of this application area. Increasing referrals from gastroenterologists, psychiatrists, and integrative medicine specialists, along with improved access to controlled clinical trial settings, continue to support growth. Advances in neuroimaging, biomarker research, and therapy-assisted protocols are enabling more precise patient selection and outcome evaluation. As interdisciplinary research expands, demand within this application segment is expected to remain strong.
By End User, Hospitals Hold the Largest Share Due to High Testing Volumes and Specialized Capabilities
The hospitals segment is projected to dominate the global psychedelic drugs market in 2026, accounting for a revenue share of 50.0%. Hospitals function as primary centers for advanced psychiatric care and complex therapeutic interventions, offering integrated services that include psychiatry, psychology, neurology, and anesthesiology. Availability of controlled clinical environments, specialized monitoring infrastructure, and multidisciplinary expertise enables hospitals to safely administer and evaluate psychedelic-assisted therapies. High patient volumes, increasing referrals for treatment-resistant mental health conditions, and participation in late-stage clinical trials support segment dominance. Hospitals also play a critical role in protocol development, therapist training, and long-term patient follow-up, which are essential for regulatory compliance and clinical validation. Academic and tertiary care hospitals, in particular, are actively involved in sponsored and investigator-initiated studies, generating additional demand. Ongoing investment in mental health infrastructure and expansion of hospital-based psychedelic research programs continue to reinforce this segment’s leading position.
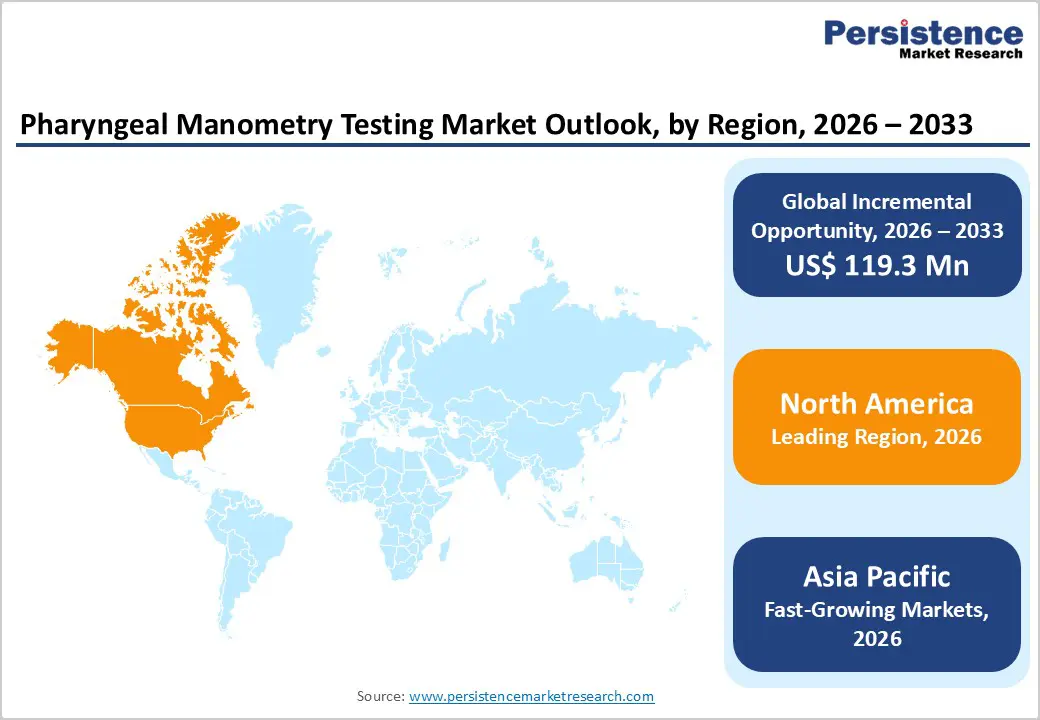
North America Pharyngeal Manometry Testing Market Trends
The North America psychedelic drugs market is expected to dominate globally with a value share of 47.6% in 2026, led primarily by the United States. The region benefits from a highly advanced healthcare and research ecosystem characterized by strong clinical trial infrastructure, early regulatory engagement, and significant private and public investment in mental health innovation. High awareness of treatment-resistant psychiatric disorders among clinicians, combined with growing acceptance of novel therapeutic paradigms, is supporting early adoption and sustained research activity.
Favorable funding environments, including venture capital participation and government-backed research grants, are accelerating development of psychedelic-assisted therapies across indications such as depression, PTSD, anxiety, and substance use disorders. North America also hosts a dense network of academic medical centers, biotechnology firms, and contract research organizations actively involved in Phase I–III trials. Strong participation in investigator-initiated studies, increasing use of digital therapeutics for patient monitoring, and expanding therapist training programs further reinforce demand. Regulatory clarity at the federal and state levels, alongside continued investment in precision psychiatry, underpins the region’s leadership position.
Europe Pharyngeal Manometry Testing Market Trends
The Europe psychedelic drugs market is expected to grow steadily, supported by increasing recognition of unmet needs in mental health care and a strong emphasis on evidence-based treatment models. Countries including Germany, the United Kingdom, France, Italy, and the Nordic region are key contributors due to robust public healthcare systems and active academic research communities. Expanding government-supported mental health initiatives and rising prevalence of depression and anxiety disorders are driving consistent interest in alternative therapeutic approaches.
European healthcare frameworks prioritize clinical validation and patient safety, encouraging structured trials of psychedelic compounds under controlled medical supervision. Cross-border research collaborations, harmonized regulatory pathways, and sustained funding for neuroscience and psychiatry programs are supporting market development. Growing integration of psychotherapy with pharmacological innovation, alongside increasing clinician education and professional training, is strengthening adoption momentum. In addition, rising patient advocacy and public discourse around mental health are reducing stigma associated with psychedelic therapies. Continued investment in clinical infrastructure, digital health platforms, and translational research is expected to sustain long-term market expansion across Europe.
Asia Pacific Pharyngeal Manometry Testing Market Trends
The Asia Pacific psychedelic drugs market is expected to register the fastest growth, with a CAGR of approximately 7.1% between 2026 and 2033, driven by rapid healthcare modernization and a large, underserved mental health patient population. Countries such as China, India, Japan, South Korea, and Australia are witnessing increasing attention toward innovative psychiatric treatments as awareness of mental health disorders improves. Rising urbanization, work-related stress, and lifestyle changes are contributing to higher diagnosis rates of depression, anxiety, and substance abuse conditions.
Expansion of clinical research capabilities, improving access to specialized psychiatric care, and growing healthcare expenditure are enhancing market readiness across the region. Government-led mental health programs and selective regulatory reforms are supporting early-stage research, while collaborations with global pharmaceutical and biotechnology companies are facilitating technology transfer and clinical expertise development. Increasing academic interest in neuropsychiatric research, coupled with gradual destigmatization of mental health treatment, is expected to accelerate adoption. Emphasis on early intervention, precision medicine, and integrated care models will continue to drive strong growth across Asia Pacific.
The global psychedelic drugs market is highly competitive, with key players such as EB Neuro S.p.A., Medtronic, Boston Scientific Corporation, Medspira, and PENTAX Medical leveraging advanced neurotechnology platforms, clinical research capabilities, and established global networks to strengthen their market presence. These companies are focused on expanding therapeutic application areas, optimizing treatment delivery and monitoring technologies, and improving clinical outcomes to support physician confidence and regulatory acceptance.
Competition is further intensified by sustained investments in clinical collaborations, participation in mental health and neuropsychiatric disorder trials, and geographic expansion into emerging markets. Continuous innovation across neurostimulation systems, digital health integration, data analytics, and precision medicine approaches is enhancing differentiation, supporting long-term market growth, and shaping the future evolution of the psychedelic drugs landscape.
Key Industry Developments:
The global pharyngeal manometry testing market is projected to be valued at US$ 232.6 Mn in 2026.
The global pharyngeal manometry testing market is driven by increasing prevalence of dysphagia and adoption of advanced high-resolution manometry systems.
The global pharyngeal manometry testing market is poised to witness a CAGR of 5.2% between 2026 and 2033.
Key market opportunities include expansion of early screening programs and integration of precision diagnostics in clinical and research settings.
EB Neuro S.p.A., Medtronic, Boston Scientific Corporation, Medspira, and PENTAX Medical are some of the key players in the pharyngeal manometry testing market.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2020 – 2025 |
|
Forecast Period |
2026 – 2033 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author