ID: PMRREP4421| 177 Pages | 15 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

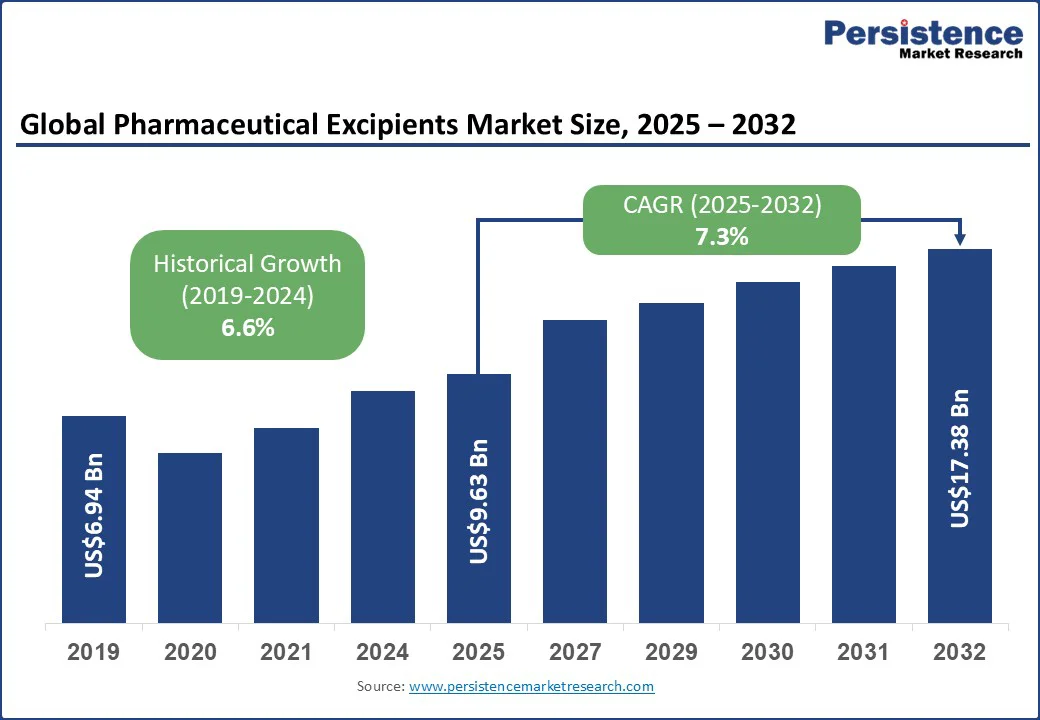
The global pharmaceutical excipients market size is likely to be valued at US$9.63 Bn in 2025, and is estimated to reach US$17.38 Bn by 2032, growing at a CAGR of 7.3% during the forecast period 2025 - 2032.
Key Industry Highlights:
| Global Market Attribute | Key Insights |
|---|---|
| Pharmaceutical Excipients Market Size (2025E) | US$9.63 Bn |
| Market Value Forecast (2032F) | US$17.38 Bn |
| Projected Growth (CAGR 2025 to 2032) | 7.3% |
| Historical Market Growth (CAGR 2019 to 2024) | 6.6% |
The formulation of biologics and advanced therapies has fueled the need for specialized excipients capable of stabilizing sensitive molecules such as monoclonal antibodies, benefiting the market. The pharmaceutical excipients market is entering a period of robust growth, driven by an increasing demand for patient-centric formulations such as orally disintegrating tablets, controlled-release drugs, and age-specific dosage forms.
The unprecedented progress in generic drug manufacturing triggered by patent expirations of blockbuster branded drugs is the primary fuel driving the pharmaceutical excipients market growth. This combination is likely to encourage pharmaceutical companies to produce high-quality generics rapidly and cost-effectively, elevating the demand for versatile, cost-efficient pharmaceutical excipients essential for formulation stability and bioavailability.
For instance, Indian pharmaceutical firms are looking forward to capitalize on an expected spike in patent expiries, small-molecule drugs worth US$63.7 Bn will go off-patent between 2025 and 2029, and an overall Loss of Exclusivity (LoE) opportunity valued at US$180 Bn across the U.S. and European Union (EU) through 2035, presenting a significant growth window for generics, especially by companies adept in complex therapies.
The dynamics intensify as the patent cliff phenomenon forces branded drugmakers to innovate, leading to increased R&D investments in novel excipients that improve drug delivery and patient compliance. This will likely have a dual effect, wherein it will boost both generic excipient consumption and high-performance excipients tailored for advanced drug delivery platforms. Supportive regulatory frameworks are fostering excipient innovation, streamlining approvals, and accelerating the adoption of multifunctional excipients, fueling the overall market growth.
In 2025, the U.S introduced tariff hikes on all imported goods and enforced steep levies specifically targeting pharmaceutical imports, with tariffs on Chinese APIs reaching up to 245%. Moreover, in September 2025, the U.S. President threatened to impose tariffs up to 200% on pharmaceutical imports, which would adversely impact access to generic drugs for low-income patients.
Such tariffs will inevitably increase costs for pharmaceutical companies reliant on global supply chains, particularly those sourcing excipients and APIs from countries such as China, India, Canada, and Mexico, which are critical suppliers in this market.
For example, nearly 40% of U.S. generic drugs depend on Chinese APIs, and the tariffs will naturally disrupt these supply chains, raising production costs and skyrocketing the price of drugs. Indian pharmaceutical exports worth US$8.7 Bn to the U.S. are also at risk due to potential reciprocal tariffs. These trade barriers will result in higher excipient costs, supply shortages, and pressure on manufacturers to overhaul sourcing strategies, which can delay product development and increase market entry costs.
The pharmaceutical excipients market stands to gain substantially from the growing emphasis on patient-centric drug delivery systems, which are forging new pathways for excipient innovation and market expansion. As pharmaceutical companies pivot toward personalized medicines, there is a soaring demand for functional excipients that enhance drug solubility, taste-masking, and controlled-release properties, especially tailored to vulnerable groups such as pediatric and elderly patients.
For example, in July 2025, Akums Drugs & Pharmaceuticals unveiled a high-strength Paracetamol Oral Suspension in India, a concentrated liquid formulation designed to deliver an adult dose in just 5 ml, making it particularly suitable for adult and geriatric patients with difficulty swallowing tablets.
This has stimulated a rise in co-processed excipients and intelligent polymers that improve drug stability and patient adherence. Industry leaders are increasingly investing in research collaborations to develop bespoke excipient solutions, accelerating formulation timelines while improving therapeutic efficacy.
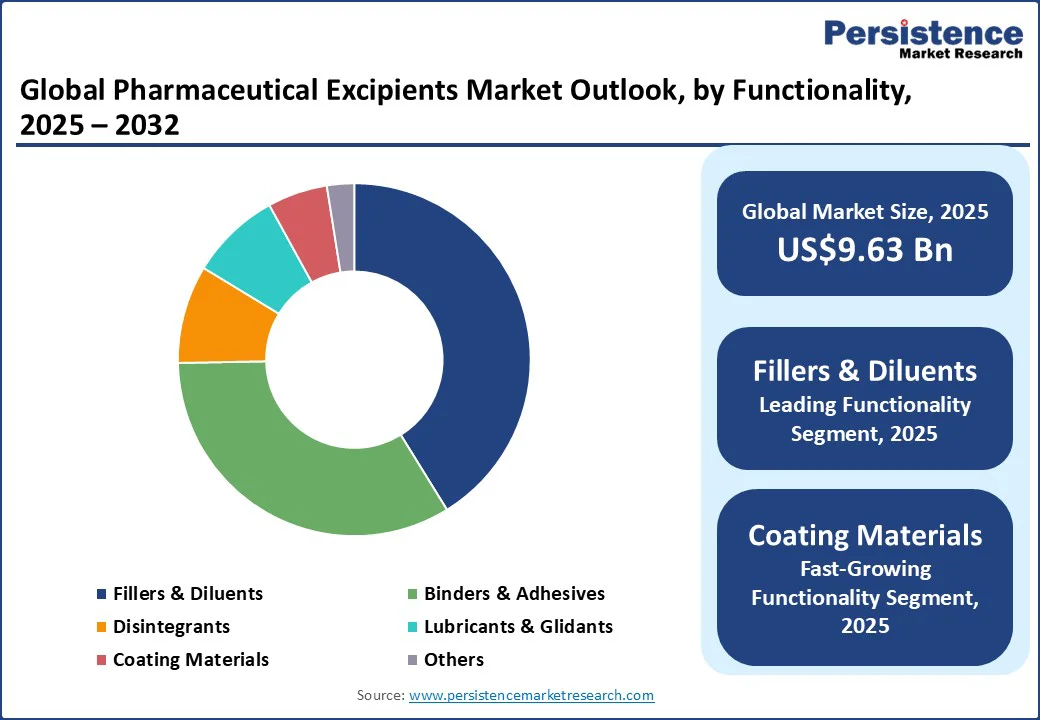
In terms of functionality, the fillers & diluents segment is expected to hold a dominant share of around 41.2% in 2025. This segment provides the necessary bulk and consistency for solid oral dosage forms such as tablets and capsules, which constitute the majority of pharmaceutical products globally.
The prolific growth in generic drugs manufacturing, especially in high-demand markets such as India and China, where excipients such as microcrystalline cellulose, lactose, and calcium phosphate are pivotal for cost-effective, high-quality tablet production. The surge in chronic disease therapies requiring sustained-release and fast-dissolving pills is likely to boost the demand even more in the coming years.
The segment with the highest CAGR through 2032 is expected to be coating materials, driven by an increasing demand for advanced drug delivery systems and patient-centric formulations. Coating materials enhance drug stability, mask unpleasant tastes, and control drug release, which are vital for compliance in pediatric, geriatric, and chronic disease patients.
The rise of biologics and targeted delivery formulations has sparked innovation in coating excipients, including film formers and enteric coatings formulated from novel polymers. Moreover, regulatory support for innovative coatings that improve product performance and shelf life is speeding up the adoption of these materials worldwide.
Solid dosage form is set to secure an estimated market share of around 68.0% in 2025, with its dominance powered by the overwhelming preference for tablets and capsules worldwide due to their ease of administration, stability, cost-effectiveness, and broad therapeutic applicability.
The growth in this sub-segment is fueled by the surge in chronic disease prevalence and generic drug production, which predominantly utilize solid forms, as well as advances in controlled-release and taste-masked formulations, enhancing patient compliance and therapeutic outcomes.
The liquid dosage form segment is projected to exhibit a leading CAGR, from 2025 to 2032, owing to the rising demand for pediatric and geriatric-friendly formulations, the growth of biologics and parenteral solutions, and an established preference among patients for easier-to-swallow medicines.
Innovations such as pharmaceutical excipients that improve solubility, stability, and flavor masking in syrups, suspensions, and injectables are broadening market horizons. This segment benefits from increasing healthcare infrastructure investments and novel drug delivery technologies that cater to the growing need for precise dosing and rapid absorption in liquid forms.
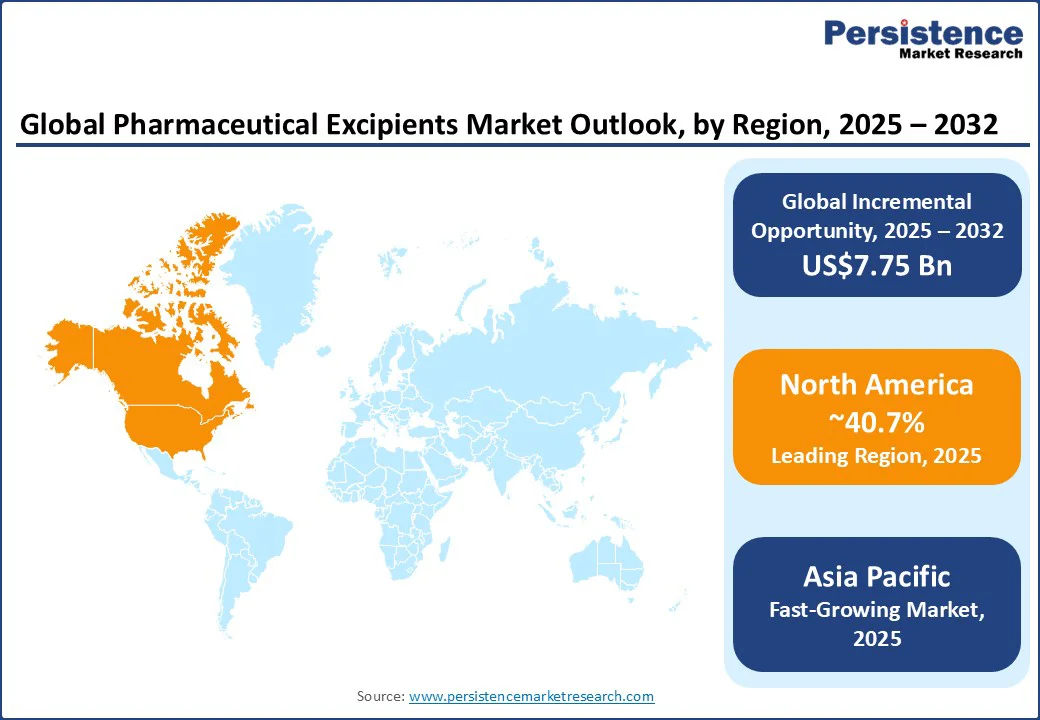
North America is anticipated to lead with a commanding 40.7% share, supported by a dense cluster of pharmaceutical innovators, well-established regulatory frameworks, and an early adoption of advanced manufacturing technologies such as continuous processing.
The leadership of the region in the research and development of biologics and specialty drugs has created a sustained demand for high-purity excipients, including parenteral-grade polysorbates and novel cellulose derivatives essential for biologics stability.
U.S. Pharma companies have been successful in capturing premium margins over the last several decades due to rigorous quality standards set by the FDA and other agencies, pushing industry players to invest heavily in R&D for multifunctional and co-processed excipients.
Asia Pacific accounts for a leading share in 2025 stands out as the fastest-growing regional market through 2032, with the highest CAGR. The explosive growth of the market is a result of the expanding pharmaceutical manufacturing capabilities and rising healthcare expenditures across India and China.
India’s status as the “pharmacy of the world” with over 300 generic drug approvals annually in the U.S. lays the foundation for an exponential rise in the demand for fillers, binders, and coating excipients in large volumes.
Favorable government policies, such as “Pharma Vision 2030” in India, are likely to incentivize local excipient production to reduce import dependency, supported by increased investments in spray-dried mannitol and other high-value excipients. Moreover, China’s growing biopharma sector and regulatory roadmap alignment with global standards are expected to attract multinational collaborations, strengthening supply chain resilience.
Europe is set to account for around 24.1% share in 2025, anchored by its mature pharmaceutical industry and stringent regulatory environment governed by the European Medicines Agency (EMA). The push toward environmentally sustainable pharmaceutical ingredients, such as plant-based binders and biodegradable polymers, stems from the clean-label policies and circular economy principles laid out by the EU.
European excipient manufacturers lead in developing multifunctional variants that reduce formulation complexity while improving drug bioavailability and stability. The increasing adoption of biosimilars and advanced drug delivery formulations by stakeholders in the region will further spur the demand for specialized excipients over the next decade.
The global pharmaceutical excipients market landscape is energized by product innovation, strategic partnerships, and advanced manufacturing capabilities, making R&D investments and collaborative ecosystems the top competition drivers.
Leading players such as BASF and DuPont are aggressively developing multifunctional and co-processed excipients tailored for complex, patient-centric drug delivery systems, addressing the growing demand for controlled-release, taste-masked, and biologics-compatible formulations.
This innovation race is reflected in the rise of biopolymers, nanoexcipients, and sustainable excipients, aligning with regulatory expectations and environmental concerns. Mergers, acquisitions, and contract manufacturing collaborations are some mainstay strategies leveraged by market leaders to diversify product portfolios, expand geographic reach, and streamline supply chains.
The pharmaceutical excipients market is projected to reach US$9.63 Bn in 2025.
The surge in generic drug manufacturing and the impending patent expirations of blockbuster branded drugs drive the market.
The pharmaceutical excipients market is poised to witness a CAGR of 7.3% from 2025 to 2032.
The growing emphasis on patient-centric drug delivery systems and the integration of artificial intelligence (AI) for excipient selection and formulation optimization are key market opportunities.
Ashland Global Holdings, Inc., BASF SE, and DuPont de Nemours, Inc. are some key players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Functionality
By End-user
By Dosage Form
By Route of Administration
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author