ID: PMRREP32316| 200 Pages | 30 Nov -0001 | Format: PDF, Excel, PPT* | Healthcare

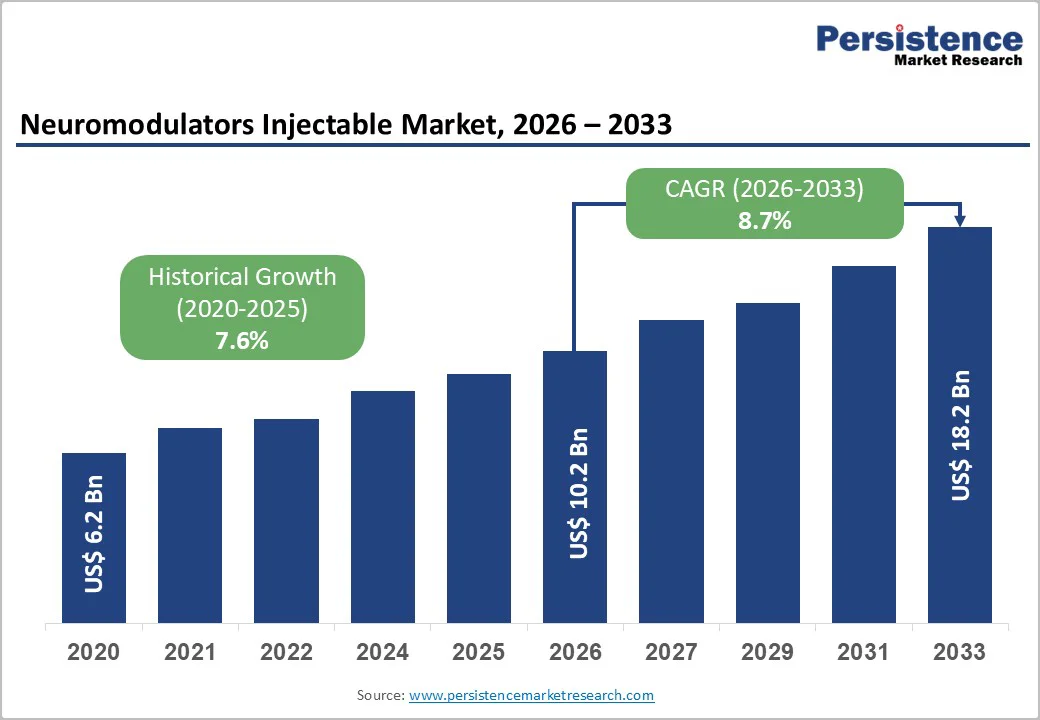
The global Neuromodulators Injectable Market is estimated to grow from US$ 10.2 Bn in 2026 to US$ 18.2 Bn by 2033. The market is projected to record a CAGR of 8.7% during the forecast period from 2026 to 2033.
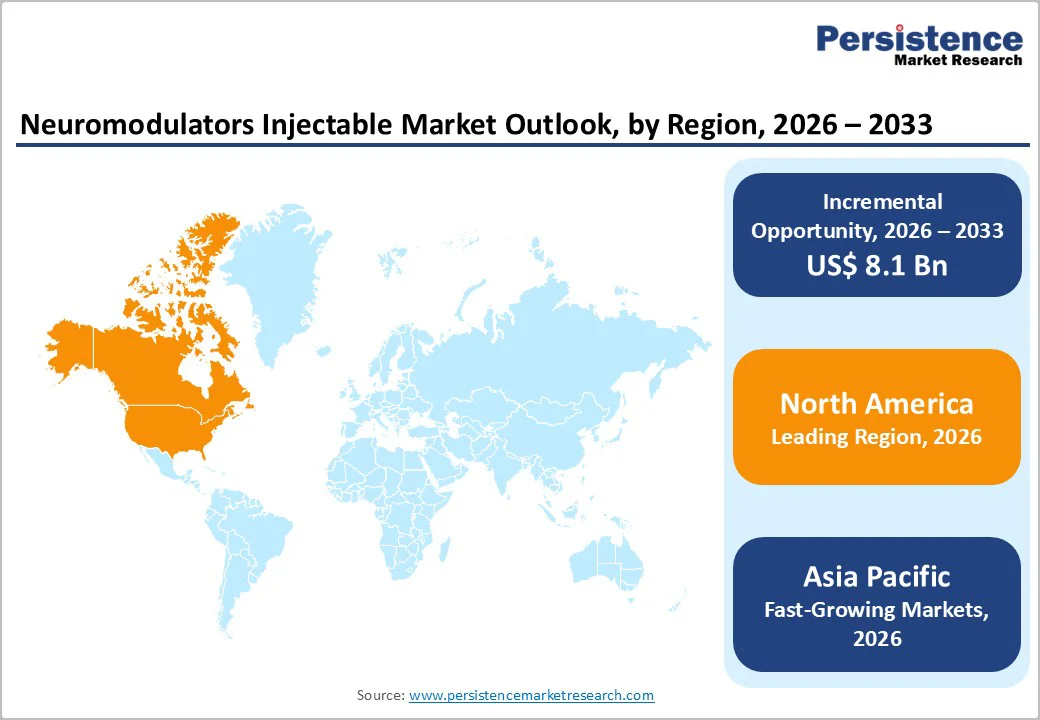
The Neuromodulators Injectable Market is expanding steadily, driven by increasing demand for cosmetic and therapeutic treatments, particularly in aesthetic procedures and neurological or urological disorders. North America dominates the market due to high consumer awareness, advanced healthcare infrastructure, and strong adoption of botulinum toxin therapies. Meanwhile, Asia-Pacific is witnessing rapid growth, supported by rising disposable incomes, expanding medical and aesthetic clinics, increasing awareness of cosmetic procedures, and growing acceptance of minimally invasive treatments. Europe also shows steady growth, fueled by mature markets and established regulatory frameworks for injectable neuromodulators.
| Global Market Attributes | Key Insights |
|---|---|
| Global Neuromodulators Injectable Market Size (2026E) | US$ 10.2 Bn |
| Market Value Forecast (2033F) | US$ 18.2 Bn |
| Projected Growth (CAGR 2026 to 2033) | 8.7% |
| Historical Market Growth (CAGR 2020 to 2025) | 7.6% |
Driver – Increasing prevalence of neurological and urological disorders treatable with neuromodulators
The increasing prevalence of neurological disorders that are treatable with neuromodulators is a significant driver for the Neuromodulators Injectable Market. Chronic migraine, defined by frequent headache days, affects an estimated about 1% of the global population, with migraine disorders overall affecting more than 1.2 billion people worldwide according to global health estimates; migraine ranks among the top causes of disability globally. Botulinum toxin type A is an approved preventive treatment for chronic migraine and several other headache disorders, contributing to greater clinical use as disease awareness rises. These rising case numbers create sustained demand for neuromodulator therapies, particularly in regions with advanced clinical practice.
Urological conditions like overactive bladder (OAB) further support market growth due to substantial global prevalence and the expansion of botulinum toxin use beyond cosmetic applications. OAB affects hundreds of millions of adults, with prevalence estimates ranging widely by age and sex but often exceeding 15–20% in adult populations and increasing sharply in older individuals. Additionally, neurogenic detrusor overactivity, a severe subset associated with conditions such as multiple sclerosis and spinal cord injury, impacts large patient groups with urinary dysfunction. Botulinum toxin injections provide a treatment option for patients refractory to conventional therapies, reinforcing clinical utilization and supporting market expansion in both therapeutic and aesthetic segments.
Restraints – High cost of neuromodulator treatments limiting accessibility in price-sensitive regions
The high cost of neuromodulator treatments is a notable restraint for the Neuromodulators Injectable Market, especially in price sensitive regions. In the United States, average botulinum toxin injections cost around USD 435 per session, and typical treatments may range from USD 300 to USD 1,000 or more depending on units used and areas treated according to data from the American Society of Plastic Surgeons; this expense is paid out of pocket for most cosmetic applications because insurers typically do not cover aesthetic procedures. In contrast, prices in markets like India range from roughly 250 to 800 per unit, translating to several thousands of rupees per session, which remains unaffordable for many patients outside urban centres. This gap between treatment cost and disposable income limits broader adoption, particularly where cosmetic and even some therapeutic uses are not reimbursed under public or private insurance schemes.
Recurrent expenditure further exacerbates accessibility issues in emerging markets. Because botulinum toxin effects typically last 3–6 months, patients often require two to four treatments per year, meaning annual spending can exceed USD 1,000–3,000 for ongoing cosmetic or therapeutic management. Data from patient surveys show that a majority of BoNT A recipients and their caregivers incur significant out of pocket costs, with more than half spending over €500 (approximately USD 540+) annually on injections alone, and many reporting costs exceeding €100 per visit before add on expenses like transportation. The cumulative financial burden, combined with limited insurance coverage for non medical indications, constrains uptake of neuromodulator therapies among lower income populations and in regions with constrained healthcare spending.
Opportunity – Development of next-generation neuromodulators with enhanced efficacy and duration
The development of next generation neuromodulators with enhanced efficacy and duration represents a major opportunity for the Neuromodulators Injectable Market. Traditional botulinum toxin products typically last three to four months, requiring frequent reinjections. However, newer formulations such as daxibotulinumtoxinA (Daxxify) have demonstrated extended effects, with clinical data showing visible results lasting six months or more, and up to nine months for some patients, compared with older products. Nearly 2,700 participants in clinical trials experienced significant wrinkle reduction within four weeks, with the prolonged duration reducing the need for repeated procedures and improving patient convenience. Longer acting neuromodulators address patient and clinician preferences for reduced treatment frequency and sustained outcomes, creating a compelling market opportunity.
In addition to extended duration, pipeline innovations like RelabotulinumtoxinA and ready to use liquid neuromodulators offer potential improvements in consistency, onset speed, and ease of use. Phase IIIb clinical data indicate rapid onset with some patients noticing effects as early as day one and sustained benefits for up to six months. These enhancements may expand clinical adoption in both aesthetic and therapeutic contexts by improving patient satisfaction and reducing resource utilization. Moreover, research into modified serotypes and molecular variants aims to tailor neuromodulators for specific clinical needs, further broadening application scope and reinforcing long term market growth.
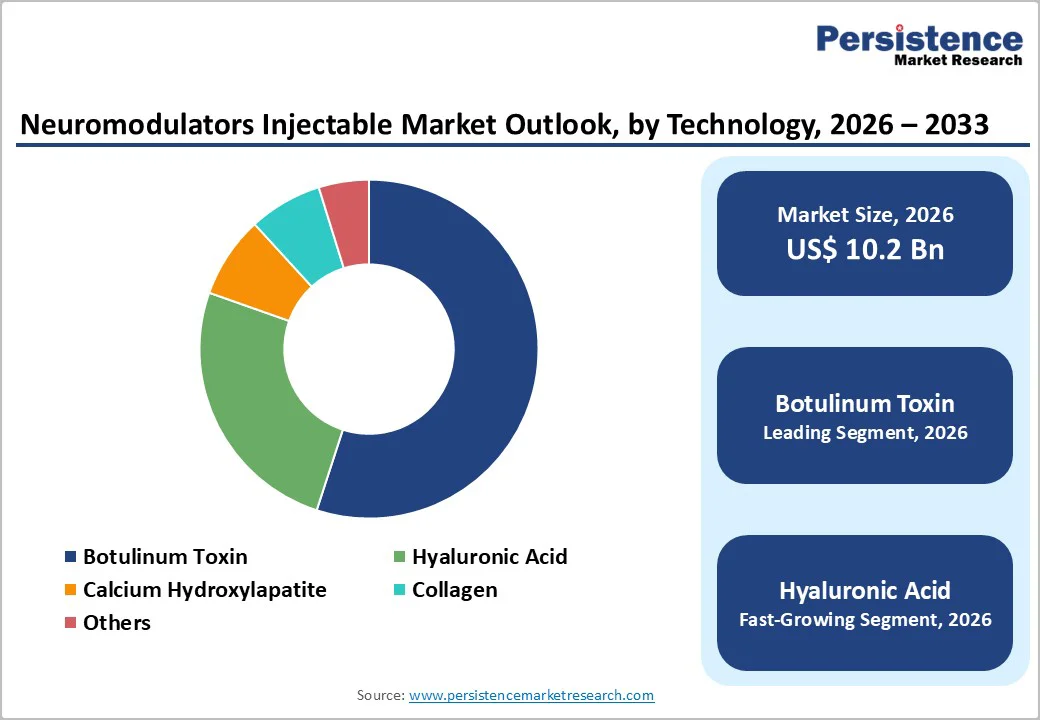
By Product Type, Botulinum Toxin Dominates the Neuromodulators Injectable Market
Botulinum Toxin dominates with 55.0% share of the global market in 2025, because it is by far the most widely used and accepted neuromodulator in both cosmetic and therapeutic applications. Globally, botulinum toxin injections accounted for millions of procedures annually, with approximately 8.8–9.8 million injections performed worldwide in 2023–2024, making it the most common non surgical injectable treatment. In the United States alone, data from professional plastic surgery sources show over 4.7 million botulinum toxin type A procedures in a single year, reflecting its broad adoption for aesthetic wrinkle reduction. Its popularity is reinforced by strong recognition of established brands like Botox and growing therapeutic uses—such as chronic migraine and muscle disorders—which further expand its clinical utilization, reinforcing its share relative to other neuromodulator types.
By Application, Frown Lines/Galbellar dominates due to high demand, proven efficacy, and patient satisfaction
Frown lines or glabellar lines dominate the neuromodulators injectable market because they represent the most common and clinically validated cosmetic indication for botulinum toxin injections, driving the highest procedural volume. Clinical and practice data show that glabellar wrinkles account for around 36–42 percent of all cosmetic botulinum toxin treatments, making them the single largest application area compared with other facial sites. This prominence is reinforced by robust placebo controlled and randomized clinical studies demonstrating significant efficacy and patient satisfaction in reducing moderate to severe glabellar frown lines, with most patients experiencing noticeable improvement within weeks of injection. The predominance of this application reflects both strong patient demand for visible rejuvenation between the eyebrows and the well established safety and effectiveness profile of botulinum toxin in this region.
North America Neuromodulators Injectable Market Trends
North America dominates the Neuromodulators Injectable Market with 40.0% share in 2025, because of exceptionally high procedural volumes and advanced healthcare systems that support widespread adoption of botulinum toxin therapies for both cosmetic and medical uses. In the United States, neuromodulator injections such as Botox, Dysport, Xeomin, Jeuveau and Daxxify reached nearly 9.5–9.8 million procedures in recent annual data, reflecting strong consumer demand for minimally invasive aesthetic treatments and reinforcing clinician confidence in the region’s clinical infrastructure. North America’s robust regulatory environment, with the U.S. FDA approving multiple neuromodulator products and indications, further encourages adoption of both aesthetic and therapeutic applications. Additionally, high disposable incomes and extensive clinic networks, including dermatology and medspa facilities, make these treatments accessible to a larger population, driving the region’s leading market share.
Europe Neuromodulators Injectable Market Trends
Europe is an important region in the Neuromodulators Injectable Market because it accounts for a significant share of global botulinum toxin procedures and revenues, reflecting strong demand for both aesthetic and therapeutic applications. European countries like Germany, France, Italy and the UK regularly report millions of botulinum toxin treatments, with Germany alone performing over 360,000 cosmetic botulinum toxin procedures in recent surveys, ranking it among the top markets worldwide. The region combines a large, aging population with rising interest in minimally invasive cosmetic enhancements, supported by widespread clinical adoption in specialty dermatology and healthcare facilities. Regulatory frameworks such as the European Medicines Agency approvals and public healthcare reimbursement for therapeutic uses also enhance clinical confidence and broader usage across EU member states, reinforcing Europe’s role as a key neuromodulator market
Asia-Pacific Neuromodulators Injectable Market Trends
Asia Pacific is the fastest growing region in the Neuromodulators Injectable Market because it combines major demographic and socioeconomic shifts that expand demand for aesthetic and therapeutic procedures. The region’s population aged 60 and over has grown rapidly, now representing about 15 percent of the total population, and is projected to rise sharply by 2050, increasing interest in anti aging and wellness treatments. At the same time, Asia Pacific accounts for a rapidly expanding share of the world’s middle class, with estimates indicating that 2 billion Asians were middle class in 2020 and that figure may exceed 3 billion by 2030, increasing discretionary spending power. These trends, combined with rising healthcare utilization and broader social acceptance of cosmetic procedures, are driving faster uptake of neuromodulator injectables across the region.
Leading applications of neuromodulators focus on aesthetic wrinkle reduction, neurological disorders, and urological treatments. Their effectiveness in improving facial appearance, managing chronic migraines, and treating overactive bladder drives clinical adoption. High safety, proven efficacy, and patient satisfaction support widespread use, enabling expanded treatment access and fueling steady growth in the global Neuromodulators Injectable Market.
Key Industry Developments:
The global Neuromodulators Injectable Market is projected to be valued at US$ 10.2 Bn in 2026.
Rising demand for cosmetic procedures, increasing neurological and urological disorders, aging population, high safety profile, and growing aesthetic awareness.
The global Neuromodulators Injectable Market is poised to witness a CAGR of 8.7% between 2026 and 2033.
Next-generation neuromodulators, emerging markets, combination therapies, AI integration, longer-lasting formulations, and expanding therapeutic applications.
Allergan (AbbVie), Ipsen Pharma, Merz Pharma, Medytox, Revance Therapeutics, Galderma.
| Report Attributes | Details |
|---|---|
| Historical Data/Actuals | 2020 – 2025 |
| Forecast Period | 2026 – 2033 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author