ID: PMRREP34955| 198 Pages | 27 Jan 2026 | Format: PDF, Excel, PPT* | Healthcare

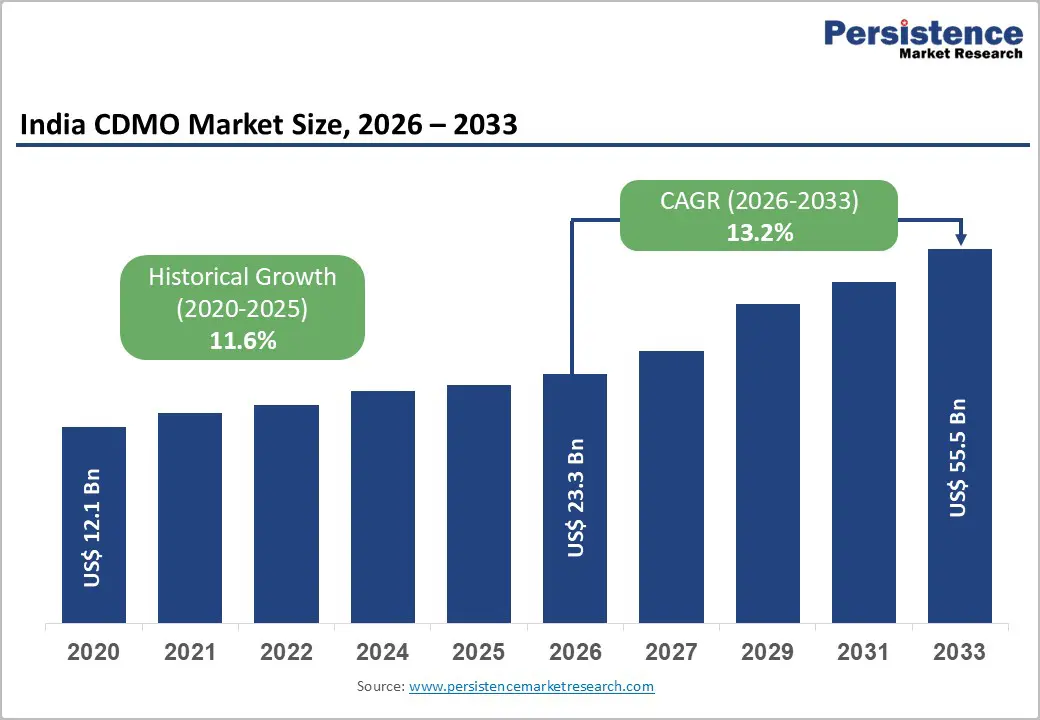
The India CDMO market size is expected to be valued at US$ 23.3 billion in 2026 and projected to reach US$ 55.5 billion by 2033, growing at a CAGR of 13.2% between 2026 and 2033.
India CDMO market has been growing steadily due to its cost-efficient manufacturing capabilities, skilled workforce, and robust pharmaceutical infrastructure. The country’s leadership in generic drug production and biosimilar development aligns with the global demand for affordable and high-quality treatments. Also, favorable government policies, such as the Make in India initiative and Production-Linked Incentive (PLI) schemes, have fueled investment in the sector. India CDMOs' adherence to international regulatory standards, coupled with their ability to provide end-to-end solutions from research and development to manufacturing, attracts global pharmaceutical companies. The rising focus on biologics and increased outsourcing of drug development also contribute to the market expansion.
| Key Insights | Details |
|---|---|
| India CDMO Market Size (2026E) | US$ 23.3 Bn |
| Market Value Forecast (2033F) | US$ 55.5 Bn |
| Projected Growth (CAGR 2026 to 2033) | 13.2% |
| Historical Market Growth (CAGR 2020 to 2025) | 11.6% |
Cost efficiency and competitive advantage is a significant driver for the India CDMO market. India’s ability to offer low-cost manufacturing without compromising on quality has positioned it as a preferred destination for pharmaceutical outsourcing.
The country benefits from lower labor costs, reduced operational expenses, and economies of scale, making it highly competitive compared to Western countries. This cost efficiency allows Indian CDMOs to produce small molecules, APIs, finished dosage forms, and even complex biologics at a fraction of the cost.
India’s affordability, coupled with its high-quality manufacturing standards, has become a key factor in driving the expansion of the CDMO market as global pharmaceutical companies seek ways to reduce production costs. The competitive advantage India holds is further strengthened by its well-established infrastructure, skilled workforce, and expertise in meeting international regulatory standards.
The rising demand for generics and biosimilars is a key driver of the India CDMO Market. As patents for blockbuster drugs expire, there is an increasing need for affordable alternatives, particularly in the form of generic drugs and biosimilars. India, being the world’s largest producer of generics is well-positioned to capitalize on this trend.
India CDMOs have developed strong capabilities in manufacturing Active Pharmaceutical Ingredients (APIs) and finished dosage forms for generics, making the country a preferred outsourcing partner for global pharmaceutical companies. Additionally, the biosimilars market is experiencing rapid growth as biologic drugs face patent expirations, and the demand for cost-effective alternatives rises.
India’s expertise in biosimilars manufacturing, along with its ability to offer high-quality products at competitive prices has made Indian CDMOs crucial players in meeting the global demand for affordable therapies. This growing demand for generics and biosimilars, combined with India’s established production infrastructure continues to drive the expansion of the country’s CDMO market.
One of the key restraining factors for the India CDMO market is the infrastructure and technology gaps in certain segments, particularly in the production of biologics and advanced therapeutics. While India’s pharmaceutical manufacturing infrastructure is well-established, some areas still lag in terms of state-of-the-art facilities required for producing complex biologics, cell and gene therapies, and other high-tech products.
India’s CDMO must invest significantly in upgrading their manufacturing capabilities to keep pace with global demands for biosimilars, monoclonal antibodies, and innovative biologic drugs. The lack of specialized facilities, equipment, and expertise in these cutting-edge areas can hinder India's ability to compete with more advanced markets.
As the demand for such high-complexity products increases, this gap may limit India growth potential in the biologics and advanced therapeutics sectors unless significant investments are made to address these technological challenges.
The rise in outsourcing by global pharmaceutical companies presents a significant opportunity for the India CDMO Market. As pharmaceutical companies worldwide face increasing pressure to reduce costs, improve efficiency, and focus on their core competencies, outsourcing manufacturing and development services has become a strategic priority.
India, with its competitive advantages such as cost-effective production, a highly skilled workforce, and compliance with global regulatory standards has emerged as a preferred destination for outsourcing. India’s CDMOs are well-positioned to capitalize on this trend by offering end-to-end services, including drug discovery, formulation development, clinical trials, and manufacturing.
Growing demand for outsourcing, coupled with India's established infrastructure and expertise, provides Indian CDMOs with the opportunity to secure long-term partnerships with global pharmaceutical companies.
Contract manufacturing services lead the India CDMO market over contract development services due to the country's established strengths in large-scale pharmaceutical manufacturing. India is a global hub for producing cost-effective Active Pharmaceutical Ingredients (APIs) and Finished Dosage Forms (FDFs), supported by its extensive manufacturing infrastructure and skilled workforce.
The rising global demand for generics, biosimilars, and vaccines has further solidified the dominance of the segment as Indian CDMOs are equipped to handle high-volume production efficiently. Additionally, the cost advantages, regulatory compliance with international standards, and the presence of export-oriented facilities make contract manufacturing the preferred choice for global pharmaceutical companies.
Small molecules dominate the product type segment in the India CDMO market due to India’s broad expertise in manufacturing Active Pharmaceutical Ingredients (APIs) and generic drugs, which are predominantly small molecules. The cost-effectiveness of producing small molecules in India, combined with its large-scale manufacturing capabilities and a well-established supply chain, makes it a preferred hub for global pharmaceutical companies.
The global demand for affordable generics has surged further boosting this segment. While biologics, biosimilars, and vaccines are growing rapidly, they require more complex infrastructure and technology, making them less dominant than small molecules.
The competitive landscape of the India CDMO market is highly dynamic, with a mix of established industry leaders and emerging players vying for dominance in various segments such as small molecules, biologics, biosimilars, vaccines, and advanced therapeutics.
Leading players like Piramal Pharma Solutions, Syngene International, Dr. Reddy’s Laboratories, Divi’s Laboratories, Lupin Limited lead the market. These companies benefit from India’s cost-effective production capabilities and regulatory expertise, enabling them to serve global markets efficiently.
Emerging players like Biocon and Piramal Pharma Solutions are focusing on specialized areas like biologics and vaccines, carving out a niche in high-complexity segments. Competition in the market is further fueled by strategic partnerships and mergers, allowing companies to enhance their capabilities and extend their global reach.
With increasing demand for biologics, biosimilars, and advanced therapeutics, India’s CDMOs are investing in technological advancements such as continuous manufacturing and bioprocessing to stay ahead. As companies expand their international presence, particularly in North America and Europe, the competitive landscape continues to evolve, driven by innovation, cost efficiency, and growing emphasis on end-to-end services.
The India CDMO market is projected to be valued at US$ 23.3 Bn in 2026.
Rising outsourcing demand, cost-competitive manufacturing, skilled workforce, strong generics/biosimilars base, expanding biologics capabilities, and global regulatory compliance.
The India market is expected to witness a CAGR of 13.2% between 2026 and 2033.
Outsourcing growth by global pharma, expansion into biologics and specialty drugs, custom API development, strategic partnerships, and advanced manufacturing technologies.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2020 - 2025 |
| Forecast Period | 2026 - 2033 |
| Market Analysis | Value: US$ Bn and Volume (if Available) |
| Country Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Service Type
By Product Type
By Scale of Operation
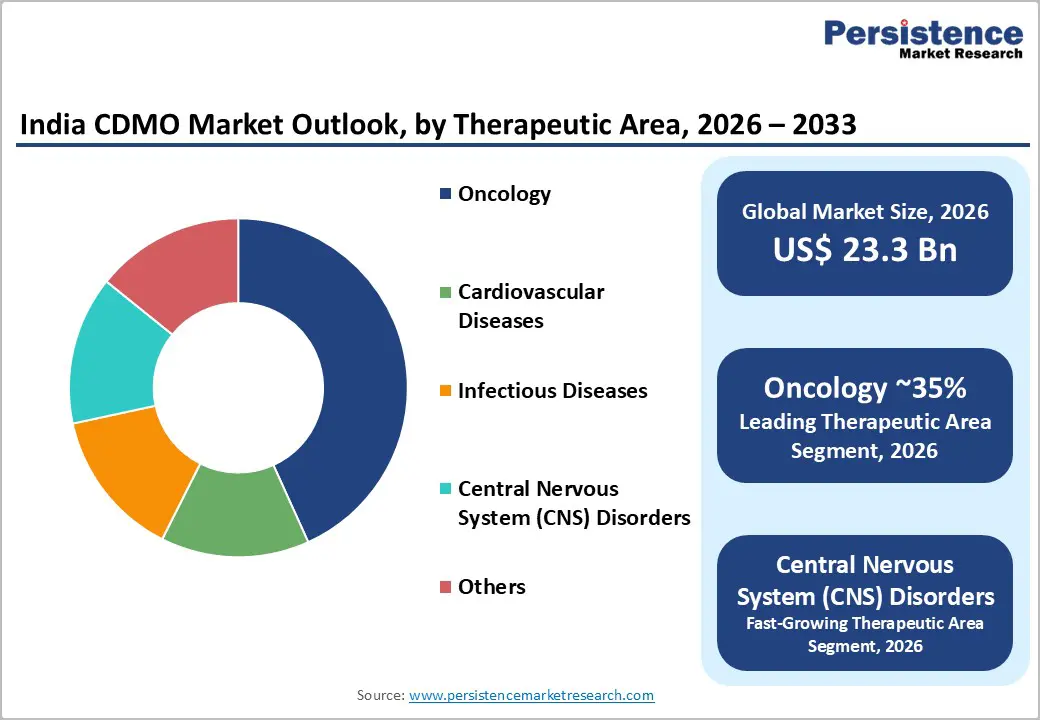
By Therapeutic Area
By Country
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author