ID: PMRREP30665| 200 Pages | 29 Nov 2025 | Format: PDF, Excel, PPT* | Healthcare

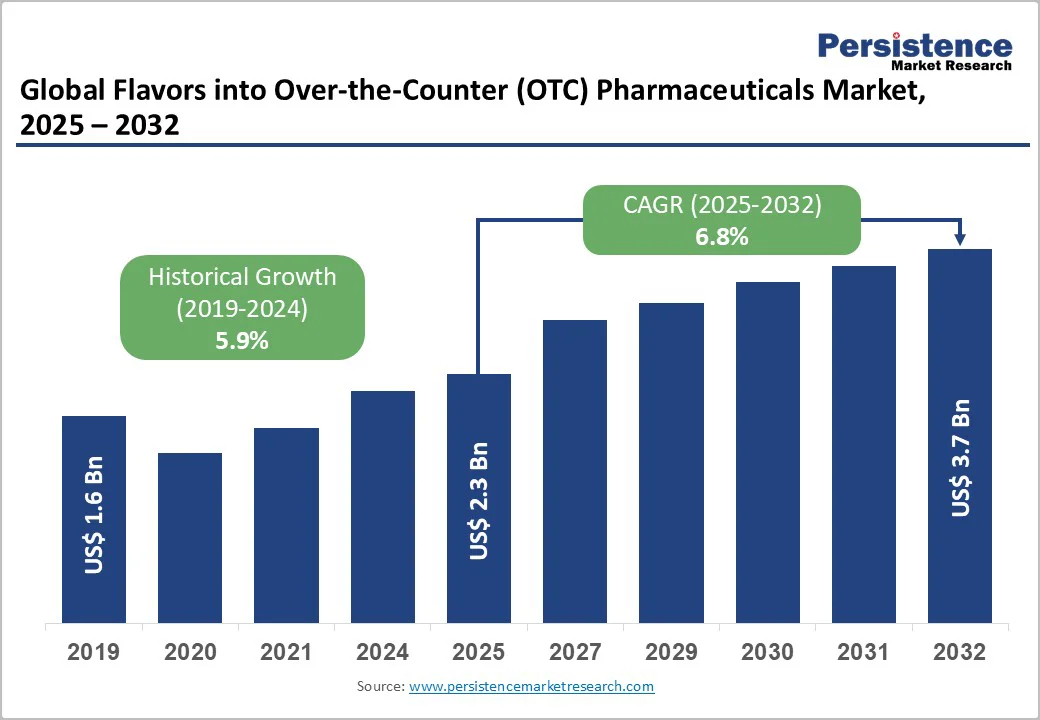
The global flavors in the over-the-counter (OTC) pharmaceuticals market size is valued at US$2.3 Bn in 2025 to US$3.7 Bn by 2032. The market is projected to record a CAGR of 6.8% during the forecast period from 2025 to 2032.
The global flavors in the over-the-counter (OTC) pharmaceuticals market is growing steadily, driven by rising consumer preference for palatable medications, flavor innovation, and increased pediatric and geriatric usage. Fruit, mint, and vanilla flavors enhance compliance and product differentiation. North America dominates the market, while Asia-Pacific is the fastest-growing region, driven by expanding healthcare access and consumer awareness.
| Key Insights | Details |
|---|---|
|
Market Size (2025E) |
US$2.3 Bn |
|
Market Value Forecast (2032F) |
US$3.7 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
6.8% |
|
Historical Market Growth (CAGR 2019 to 2024) |
5.9% |
The growing demand for flavored tablets in the over-the-counter (OTC) pharmaceuticals market is driven by rising patient preference for palatable and easy-to-consume medications. According to the U.S. Food and Drug Administration (FDA), improving taste and swallowability enhances medication adherence, especially among children and older adults. Studies published on PubMed indicate that approximately 64% of pediatric patients refuse unflavored or bitter medicines, underscoring the need for taste-masked or flavored formulations. Additionally, oral solid dosage forms such as tablets and chewables dominate OTC product sales, accounting for nearly 40% of the market, as per data cited by the U.S. National Library of Medicine. The shift toward consumer-friendly, fruit- or mint-flavored OTC tablets, especially vitamins, antacids, and pain relievers reflects broader trends in self-medication and preventive health. As flavor technology advances, pharmaceutical companies are increasingly adopting natural and sugar-free flavor systems to enhance compliance and improve the overall user experience.
A major restraint in the flavors into the OTC pharmaceuticals market is the lack of awareness and poor availability of OTC drugs, especially in low- and middle-income regions. Studies show that in a rural Indian survey, only 15.5 % of respondents had “excellent” knowledge of OTC drug safety, while 18.0 % were rated “poor” 53.5 % were in the “fair/good” range. Meanwhile, a global review of essential medicines indicated that in low-income countries the median availability of any product type was only 43.3 % in the public sector and 66.7 % in the private sector. This means a significant portion of the population cannot reliably access even basic non-prescription medicines. Such gaps in availability, combined with limited patient education, reduce the potential uptake of flavored OTC tablets, as consumers either cannot find them or don’t recognize their relevance or safe use.
The push toward natural and sugar-free flavor systems represents a compelling opportunity in the flavors for the OTC pharmaceuticals market. In India, the Indian Council of Medical Research’s recent Dietary Guidelines recommend a maximum intake of just 20–25 g of sugar per day and caution against ultra-processed and high-sugar foods. Meanwhile, the Food Safety and Standards Authority of India has reinforced that non-sugar sweeteners (NSS) are not endorsed for weight control, highlighting consumer interest in sugar reduction but regulatory caution. On the regulatory front, the U.S. Food & Drug Administration (FDA) defines “natural flavors” as extracts derived from spices, fruits, herbs or vegetables, whose primary role is flavoring rather than nutrition.
Given the growing consumer demand for ‘clean-label’, low-sugar, palatable medications, especially among pediatric and geriatric users formulating OTC tablets with natural flavor profiles and minimal added sugar positions manufacturers strongly. Adopting sugar-free or reduced-sugar flavor systems in OTC tablets can enhance user compliance, meet evolving regulatory expectations and align with broader public-health trends towards lower sugar reliance.
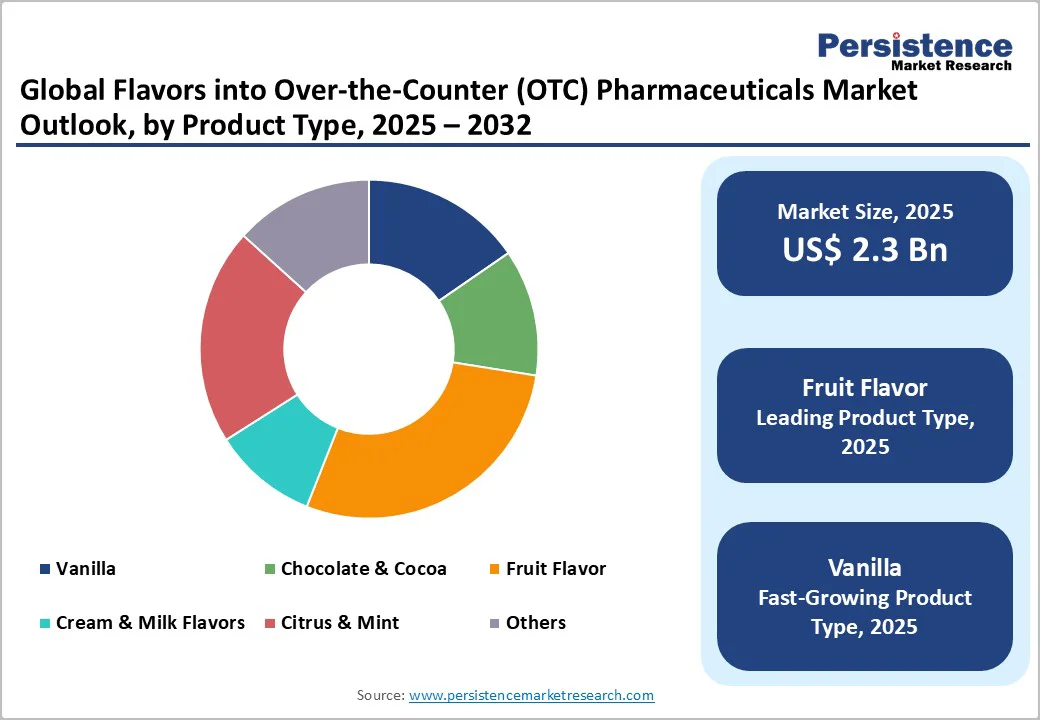
Fruit Flavor dominates the market with 28.5% share in 2025, because they significantly boost patient acceptability and compliance. Numerous studies show that taste is a pivotal factor in medication uptake: for children, sweet, fruit-flavored formulations are clearly preferred, with one Indian study finding that 57% of children preferred fruit-flavored syrups. In older adults too, flavored oral liquid medications were shown to be better accepted than unflavored ones.
Moreover, research demonstrates that 64% of studies report taste-related rejection of medications among pediatric patients, underlining the critical role of palatable flavor profiles. Thus, fruit flavors become the preferred choice because they mask bitterness, appeal to a broad age range, and foster better adherence, making them the dominant product type in the market.
Liquid formulations dominate the Flavors into Over-the-Counter (OTC) Pharmaceuticals Market due to their ease of administration, better taste masking, and suitability for all age groups. According to the U.S. FDA, liquid oral dosage forms are particularly recommended for children and the elderly who face difficulty swallowing tablets or capsules. A 2022 study in BMC Pediatrics reported that nearly 32% of caregivers preferred flavored liquid medicines for children due to improved acceptability and accurate dosing. Moreover, flavored syrups, suspensions, and drops allow for flexible dosing and faster absorption compared to solids.
The World Health Organization (WHO) also recognizes liquid oral formulations as essential in pediatric medicine, ensuring compliance and therapeutic effectiveness. Thus, their palatability and convenience make liquids the dominant form in flavored OTC pharmaceuticals.
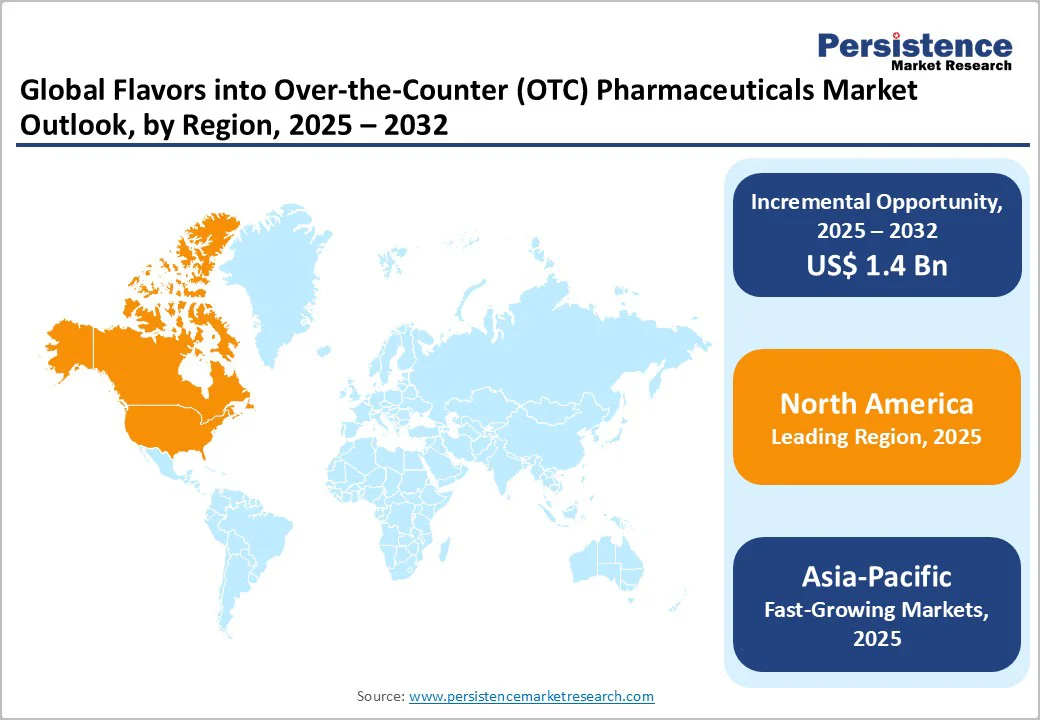
North America region dominates the global market with 39.2% share in 2025, due to its strong consumer awareness, high OTC adoption, and advanced retail infrastructure. According to the Consumer Healthcare Products Association (CHPA), nearly 81% of U.S. adults use OTC medicines as their first treatment option for minor ailments, reflecting a mature self-medication culture. The region’s regulatory framework, led by the U.S. Food and Drug Administration (FDA), ensures stringent quality and labeling standards that encourage trust in flavored OTC formulations. American households spend around USD 338 annually on OTC products and make over 26 pharmacy trips per year, demonstrating strong purchase frequency.
Combined with extensive product innovation in flavored syrups, gummies, and chewable, these factors firmly establish North America as the leading regional market.
The Asia Pacific region is emerging as the fastest-growing segment in the flavors-into-OTC pharmaceuticals space due to several converging factors. First, the region is facing a sharp rise in self-care and OTC usage: for example, in Southeast Asia over 70 % of outpatient treatments are initiated without physician consultation. (WHO / UNICEF) This indicates a broad base of consumers turning to OTC solutions. Second, regulatory shifts such as increased OTC approvals in China, e.g., 5,041 OTC drugs approved by the Chinese regulator as of September 2022 are expanding product availability.
Moreover, the ageing population and rising chronic-condition burden boost demand for easy-to-take, flavored OTC tablets and syrups. These dynamics have a strong self-medication culture, regulatory enablement, and unmet access to healthcare, making the Asia Pacific a key growth frontier for flavored OTC pharmaceuticals.
Europe stands out as an important region in the flavors-into-OTC pharmaceuticals market thanks to a mature self-care culture, regulatory support and broad consumer adoption. For instance, in 2022, Europeans purchased about 8.5 billion packs of non-prescription medicines and 1.3 billion packs of vitamins/minerals, indicating strong usage. Further, around 34.3% of EU adults (based on the European Commission Health Interview Survey) report engaging in self-medication. Also, non-prescription medicines account for 30% or more of retail pharmaceutical spending in several EU nations (e.g., Spain 36%).
These factors, high volume of OTC use, favorable cost-sharing structures and strong pharmacy networks make Europe a significant and strategically relevant market for flavored OTC pharmaceutical solutions.
The global Flavors into over-the-counter (OTC) pharmaceuticals market is expanding steadily, driven by rising consumer preference for palatable medicines, pediatric and geriatric demand, and flavor innovation. Manufacturers focus on natural, sugar-free formulations and improved taste masking. North America leads the market, while Asia-Pacific grows fastest due to expanding healthcare access and self-medication awareness.
The global Flavors into over-the-counter (OTC) pharmaceuticals market is projected to be valued at US$ 2.3 Bn in 2025.
Rising road accidents, aging population, improved emergency response systems, and technological advancements in stretcher design drive the global Flavors into Over-the-Counter (OTC) Pharmaceuticals Market.
The global Flavors into over-the-counter (OTC) pharmaceuticals market is poised to witness a CAGR of 6.8% between 2025 and 2032.
Growing adoption of electric and hydraulic stretchers, telemedicine integration in ambulances, and expanding healthcare infrastructure create key market opportunities.
Stryker Corporation, Medline Industries, LP, Narang Medical Limited, FU SHUN HSING TECHNOLOGY CO., LTD, Ferno‑Washington, Inc., Zhangjiagang New Fellow Med Co., Ltd., and Others.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn Volume: Tons |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Product Type
By Form
By Nature
By End-use Application
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author