ID: PMRREP14317| 159 Pages | 27 Oct 2025 | Format: PDF, Excel, PPT* | Healthcare

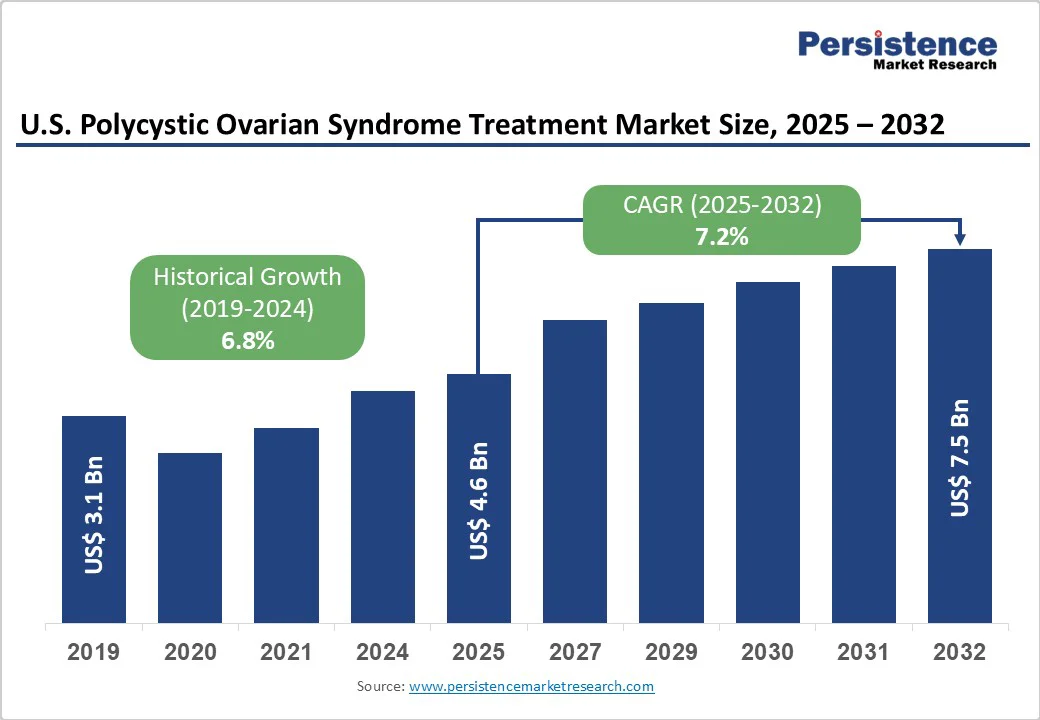
The U.S. polycystic ovarian syndrome treatment market size is likely to be valued at US$4.6 Billion in 2025 and is estimated to reach US$7.5 Billion in 2032, growing at a CAGR of 7.2% during the forecast period 2025-2032, driven by an increasing recognition of the long-term health implications of PCOS and developments in metabolic as well as hormonal therapies.
| Key Insights | Details |
|---|---|
|
U.S. Polycystic Ovarian Syndrome Treatment Market Size (2025E) |
US$4.6 Bn |
|
Market Value Forecast (2032F) |
US$7.5 Bn |
|
Projected Growth (CAGR 2025 to 2032) |
7.2% |
|
Historical Market Growth (CAGR 2019 to 2024) |
6.8% |
Untreated PCOS has been linked to long-term complications such as type 2 diabetes, cardiovascular disease, and endometrial cancer. A 2024 National Institutes of Health (NIH) report indicated that nearly 40% of women with PCOS develop metabolic syndrome before age 40, highlighting an urgent requirement for early screening and preventive care.
This trend is enabling pharmaceutical and digital health companies to introduce long-term disease management programs, including metabolic and hormonal therapies, beyond reproductive years. Insurance-backed preventive care packages are also gaining traction to reduce chronic disease risk among women with PCOS.
PCOS treatment in the U.S. is increasingly moving beyond hormonal regulation to include mental health, nutrition, and lifestyle interventions. Clinics such as Allara Health and Kindbody are integrating dietary coaching, cognitive therapy, and personalized exercise plans alongside medical treatment.
This holistic model not only improves fertility and hormonal balance but also refines mental well-being, addressing the anxiety and depression often associated with PCOS. The surging acceptance of multidisciplinary and digital wellness platforms presents a key growth driver for integrated PCOS care.
Although oral contraceptives are widely used to control menstrual irregularities and hyperandrogenism in PCOS, they can worsen lipid imbalances in women with pre-existing dyslipidemia. Recent studies published in The Journal of Clinical Endocrinology & Metabolism in 2024 indicated that certain estrogen-progestin combinations elevate LDL cholesterol and triglyceride levels, raising cardiovascular risks.
It limits their suitability for women who already face metabolic complications linked to PCOS. Hence, clinicians are increasingly cautious in prescribing them, often requiring lipid profiling and regular monitoring, which slows down treatment adoption and adds to the total management cost.
Spironolactone and similar androgen-blocking drugs remain effective for reducing acne and hirsutism but pose significant reproductive safety challenges. These drugs can cause fetal abnormalities if taken during pregnancy, compelling physicians to pair them with reliable contraception or restrict their use in women actively trying to conceive.
According to a 2023 review by the American College of Obstetricians and Gynecologists, nearly 25% of women discontinue these medications due to safety or compliance concerns. This narrow therapeutic window and the requirement for strict contraceptive measures hinder broad clinical adoption, specifically among reproductive-age women seeking fertility-friendly treatments.
The increasing focus on managing insulin resistance in women with PCOS is pushing demand for insulin-sensitizing medications such as GLP-1 receptor agonists and natural compounds, including myo-inositol. These treatments help improve ovulation, weight control, and metabolic balance. A 2024 study by the American Journal of Obstetrics and Gynecology showed that GLP-1 users with PCOS experienced nearly 20% improvement in insulin sensitivity within six months.
Myo-inositol supplements are also being widely recommended by endocrinologists for patients intolerant to metformin, promoting non-pharmacological management. This expanding adoption of both pharmaceutical and nutraceutical insulin-sensitizers presents a key growth opportunity in the U.S. PCOS treatment landscape.
In fertility treatment for PCOS, letrozole has increasingly replaced clomiphene citrate as the first-line ovulation-inducing drug. Clinical research supported by the American Society for Reproductive Medicine in 2023 confirmed that letrozole leads to high live birth and ovulation rates with fewer side effects.
Fertility clinics across the U.S., such as Shady Grove Fertility and CCRM, now primarily prescribe letrozole for anovulatory infertility linked to PCOS. This shift toward new, more effective therapeutics is opening avenues for pharmaceutical companies to develop next-generation aromatase inhibitors and combination protocols customized to PCOS-related infertility.
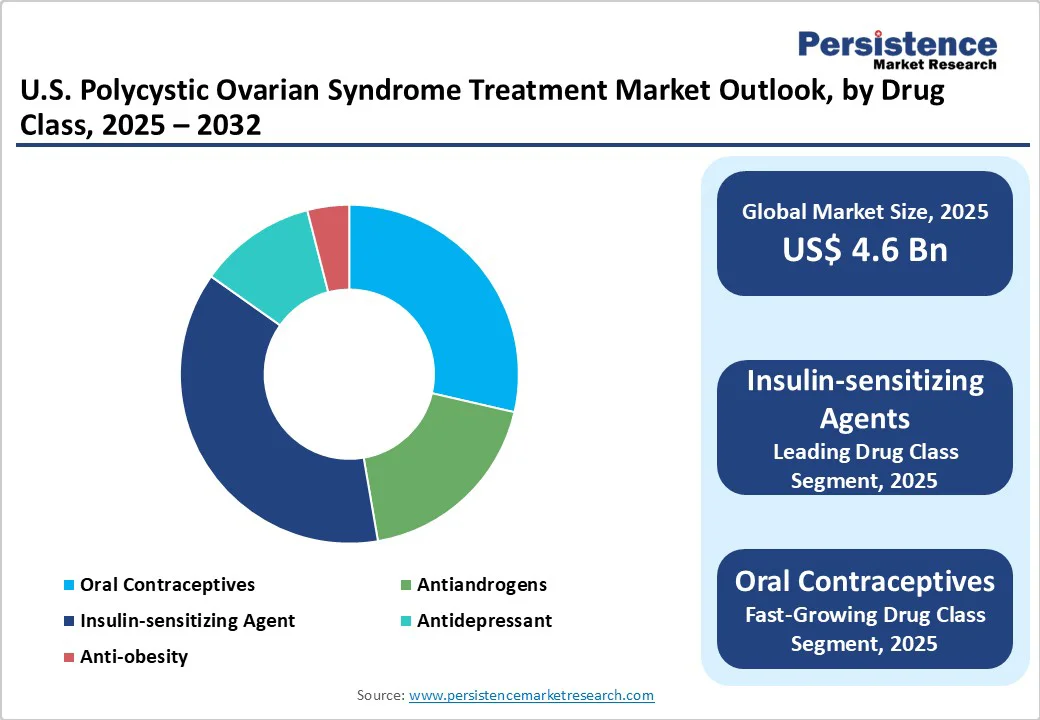
Insulin-sensitizing agents lead with approximately 37.5% of the market share in 2025, as insulin resistance is a core metabolic feature in most women with PCOS. Metformin and GLP-1 agonists help improve ovulation, reduce androgen levels, and promote weight management. The recent rise of GLP-1 therapies such as semaglutide and tirzepatide has further strengthened this category by providing improved weight loss and metabolic control, leading clinicians to prioritize them for long-term disease management.
Oral contraceptives are predicted to gain traction through 2032 as they effectively regulate menstrual cycles, lower testosterone levels, and manage acne and excess hair growth. They are especially favored in women who are not seeking pregnancy. Modern formulations with drospirenone and low-dose estrogen are also gaining preference, as they minimize the risk of thrombotic events while maintaining hormonal balance and improving cosmetic symptoms.
In 2025, laparoscopic ovarian drilling is predicted to account for nearly 69.2% of the market share as it restores ovulation while avoiding the risk of multiple pregnancies seen with ovulation-inducing drugs. The procedure selectively destroys ovarian stroma to lower androgen production and improve follicular response. Recent developments in minimally invasive techniques, such as single-port laparoscopy and laser-assisted drilling, have made the surgery safe and more precise.
Ovarian wedge resection is regaining attention as a surgical alternative for women resistant to hormonal or medical therapies. Modern laparoscopic methods have minimized complications such as adhesion formation that were common with old techniques. This renewed interest is supported by clinical studies showing that controlled resection helps normalize ovarian morphology and improve ovulation outcomes. Some U.S. fertility centers are experimenting with modified wedge resections that use energy-based devices for better precision. This trend reflects a surging preference for targeted surgical correction among women who do not respond to medications such as clomiphene or letrozole.
Hospital pharmacies will likely dominate with a share of around 66.7% in 2025 as these ensure accurate dosing, lab monitoring, and integration with other hospital-based services such as endocrinology and fertility care. Hospitals also have direct access to new formulations and combination therapies under clinical trials. For instance, multiple GLP-1 therapies for PCOS-related obesity are dispensed primarily through hospital-linked pharmacies to ensure safe initiation and monitoring of side effects such as hypoglycemia or gastrointestinal discomfort.
Online providers are anticipated to remain in the second position through 2032 due to increasing telemedicine adoption and the requirement for privacy in women’s health management. Platforms such as Allara Health and Nurx allow virtual consultations and home delivery of oral contraceptives, metformin, and nutritional supplements, including myo-inositol. A 2024 survey revealed that over 35% of U.S. women with PCOS use at least one telehealth platform for prescription renewals.
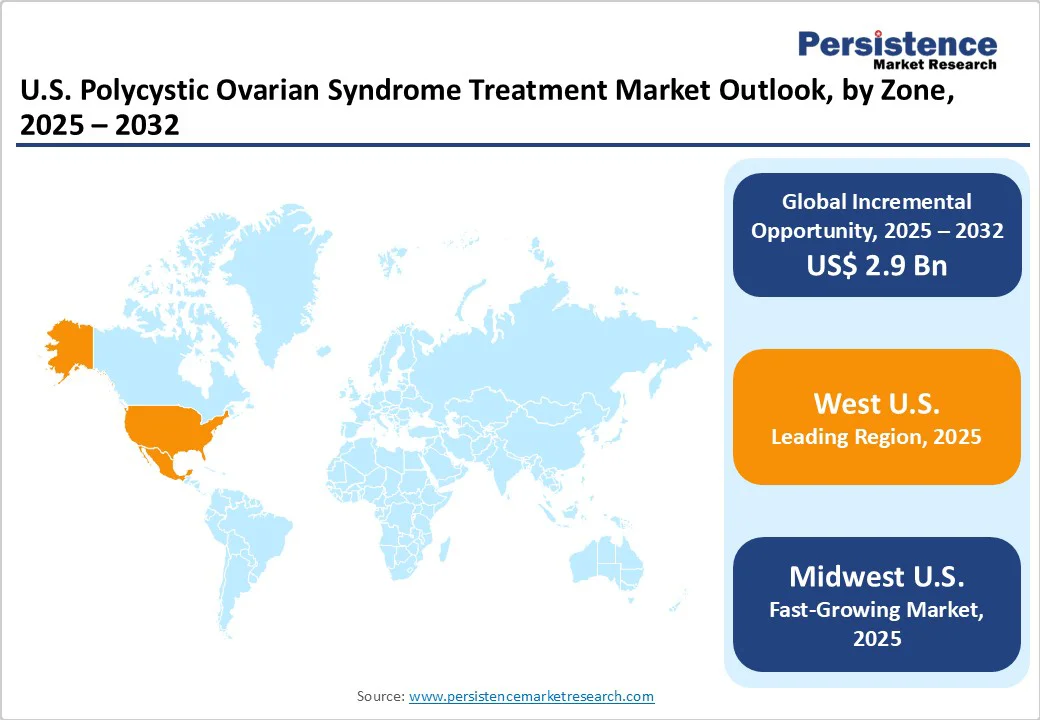
In the Western U.S., treatment for PCOS is advanced and well-integrated, owing to the presence of leading research and teaching hospitals. States such as California and Washington have several multidisciplinary PCOS clinics that combine endocrinology, fertility, dermatology, nutrition, and mental health care. For instance, the University of California, San Francisco (UCSF) and the University of California, Los Angeles (UCLA) Health systems provide specialized PCOS programs that include lifestyle counseling, hormone management, and fertility treatments under one roof.
Several women in urban areas such as Los Angeles, San Francisco, and Seattle also have access to GLP-1 therapies, including semaglutide for metabolic management. However, access is more limited in rural parts of the West, where women often rely on telehealth consultations rather than in-person specialist care.
In the Midwestern U.S., treatment is mainly centered on large university hospitals and fertility clinics in cities such as Chicago, Minneapolis, and St. Louis. Institutions, including Chicago Medicine and the Cleveland Clinic, provide structured PCOS programs that emphasize hormonal regulation, fertility care, and metabolic management.
Private clinics across Illinois, Indiana, and Ohio follow similar protocols, delivering medications such as metformin, letrozole, and hormonal contraceptives alongside nutritional counseling. However, rural and semi-urban areas in the Midwest still face challenges with specialist availability, and women often depend on primary care physicians or OB-GYNs for PCOS diagnosis and management rather than endocrinologists.
In the Southeastern U.S., PCOS care is becoming more patient-centric with the rise of telehealth and boutique hormone clinics. States such as Georgia, Florida, and Texas have seen an increase in dedicated women’s health centers focusing on PCOS and metabolic health. For example, clinics such as WomenBlossom in Georgia and PCOS Sisters provide both in-person and online consultations, combining hormonal therapy with modern options, including GLP-1 injections.
University-based centers such as UTHealth in Houston integrate dietitians and mental health specialists into PCOS management. Still, access disparities persist in rural areas of the Southeast, where patients depend heavily on virtual care platforms due to limited local endocrinology or fertility services.
The U.S. polycystic ovarian syndrome treatment market is diverse, with both pharmaceutical companies and digital health players actively involved. Pharma giants such as Novo Nordisk and Eli Lilly are gaining impetus in the PCOS field through their GLP-1 receptor agonists, Ozempic (semaglutide) and Mounjaro (tirzepatide). Although these drugs are primarily approved for type 2 diabetes and obesity, U.S. clinicians are now prescribing them off-label to help women with PCOS manage weight and metabolic complications.
The U.S. polycystic ovarian syndrome treatment is projected to reach US$4.6 Billion in 2025.
Rising insulin resistance among women and increasing awareness of long-term health risks are the key market drivers.
The U.S. polycystic ovarian syndrome treatment is poised to witness a CAGR of 7.2% from 2025 to 2032.
Expansion of integrated digital health platforms and shift toward GLP-1 therapies are the key market opportunities.
Pfizer Inc., Teva Pharmaceutical Industries, and Novartis International AG are a few key market players.
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Bn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Drug Class
By Surgery
By Distribution Channel
By Zone
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author