ID: PMRREP28351| 189 Pages | 30 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

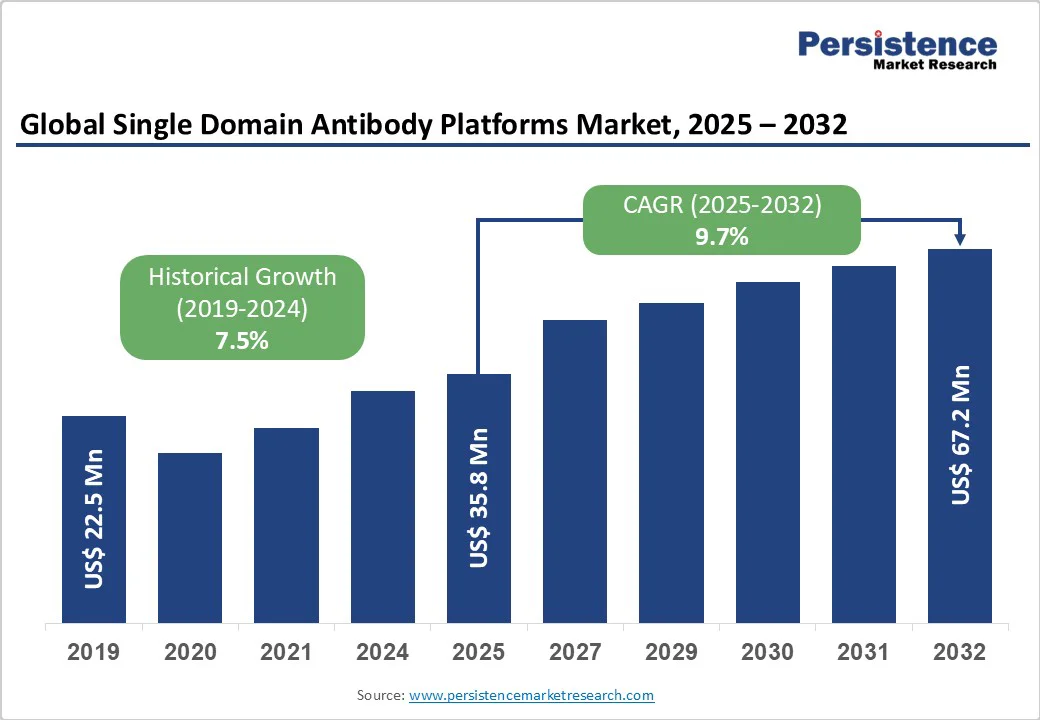
The global single domain antibody platforms market size is likely to value US$ 35.8 Mn in 2025 and is projected to reach US$ 67.2 Mn by 2032 growing at a CAGR of 9.7% during the forecast period from 2025 to 2032.Single domain antibody platform’s role in therapeutic development is anticipated to propel growth at a significant rate throughout the forecast period.
| Key Insights | Details |
|---|---|
|
Single Domain Antibody Platforms Market Size (2025E) |
US$ 35.8 Mn |
|
Market Value Forecast (2032F) |
US$ 67.2 Mn |
|
Projected Growth (CAGR 2025 to 2032) |
9.7% |
|
Historical Market Growth (CAGR 2019 to 2024) |
7.5% |
Single domain antibodies are highly versatile and adaptable, thereby making them an invaluable tool for medical research, disease detection and treatment, and innovations in biotechnology. The medical companies are actively collaborating with research institutes, biopharmaceutical firms, and Clinical Research Organizations (CROs) to find novel applications to develop innovative products. There is an increasing focus on developing and upgrading key healthcare technologies, along with the introduction of new products and services. For instance, in December 2023, GenScript Biotech Corporation announced the expansion of its collaboration with Roche, a partnership that began in 2011 under a Laboratory Service Agreement. The expanded collaboration aimed to further drive innovation across GenScript’s portfolio of life science research tools and services globally.
Manufacturers are increasing investments in research and development to bolster innovation. They are prioritizing developing economies as these countries are showing fast growth in the single domain antibodies market share. The potential for outsourcing biotech projects has increased owing to the availability of skilled labor at affordable prices in developing nations such as China and India.
As biologics progress through commercialization and clinical trials, regulatory requirements are likely to become more stringent. The approval of biological products in the U.S. requires a Biologics License Application (BLA). The product, as well as the process, must be validated and exclusively monitored against Critical Quality Attributes (CQAs) throughout the manufacturing process.
To gain approval, regulatory bodies must approve the end product as well as the process, CQAs, antibodies, consumable cell lines, and equipment that were used during production. The extended time associated with waiting for regulatory approvals is likely a challenge. It is necessary to use more than one animal source, as camelid species may not be readily available to all researchers due to animal husbandry because it necessitates specialized facilities. Analyzing the consistency of immunization processes like DNA immunization is also crucial to avoid the heterogeneity of immune responses. This can emerge as a key challenge to maintain process efficiency.
SbAds have shown promise in targeting several viruses, including HIV, influenza, SARS-CoV-2, and Ebola. They can bind to viral proteins with high specificity, potentially leading to the development of broad-spectrum antiviral therapies that can treat an array of viral infections.
Single-domain antibody platform offers rapid response solutions for new and emerging infectious diseases owing to its quick production processes. Their stability and small size make them ideal for rapid deployment in response to pandemics or outbreaks.
SdAbs may be affordable and swift to generate compared to conventional monoclonal antibodies in preventive as well as therapeutic applications for viral infections. The requirement for antibody-based therapeutics is hence projected to rise across the globe.
Camelids are the primary biological source of single-domain antibodies (sdAbs), accounting for around 92.3% of production. These animals possess a distinctive immune system that generates heavy-chain antibodies, from which sdAbs are derived. The small size of camelid-based sdAbs allows them to penetrate tissues more effectively and bind to hard-to-reach epitopes, making them highly valuable for targeting complex diseases. Their inherent stability enhances reliability while also simplifying handling, storage, and transport.
Camelid sdAbs are developed across multiple therapeutic indications and can be engineered into diverse formats of targeted therapeutics. Furthermore, their high specificity and compact structure make them ideal for diagnostic use, as seen in rapid infectious disease testing and medical imaging applications, including improved tumor detection.
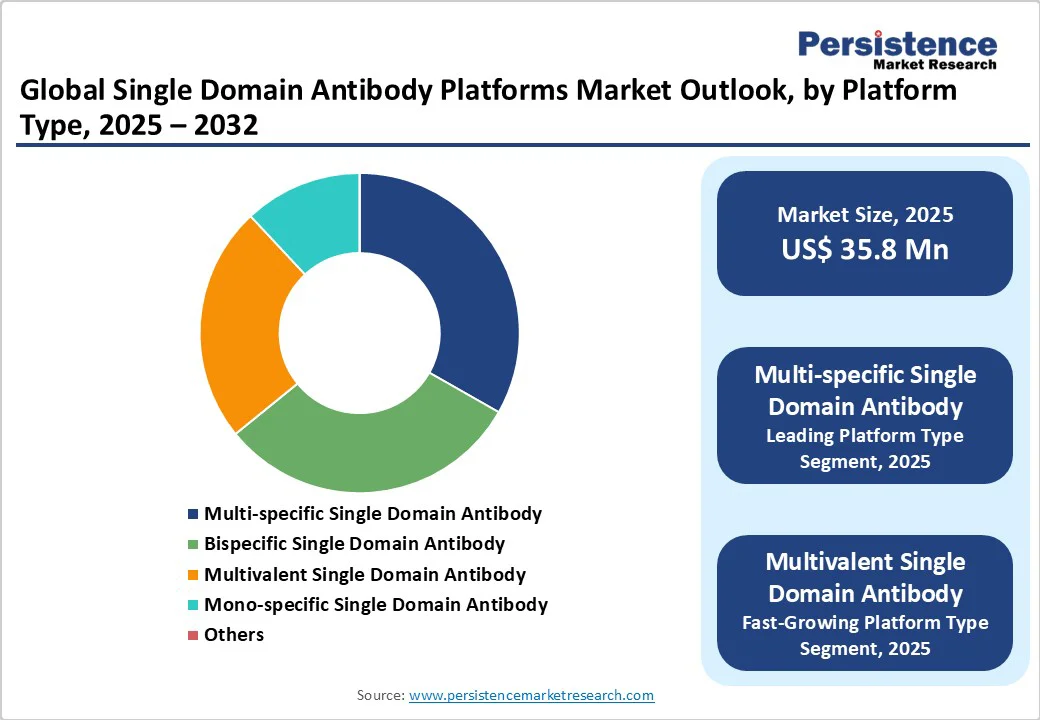
Therapeutic development represents one of the most prominent applications of single-domain antibody (sdAb) platforms, accounting for 45.0% share. SdAbs are gaining traction in this space due to their versatility, which allows them to bind multiple targets at once, thereby enhancing therapeutic potential. In addition, sdAb platforms support cost-effective and rapid manufacturing, making them more affordable and scalable compared to traditional monoclonal antibodies—an advantage for both large-scale and low-cost therapeutic applications. Their use is expanding into diverse areas, including Enzyme Replacement Therapy (ERT), where they improve enzyme delivery to lysosomes, and vaccine development, where they help boost immune responses. Moreover, their ability to cross the blood-brain barrier makes sdAbs especially valuable in addressing neurological disorders and in gene therapy applications, where they facilitate the targeted delivery of genetic material to specific tissues or cells.
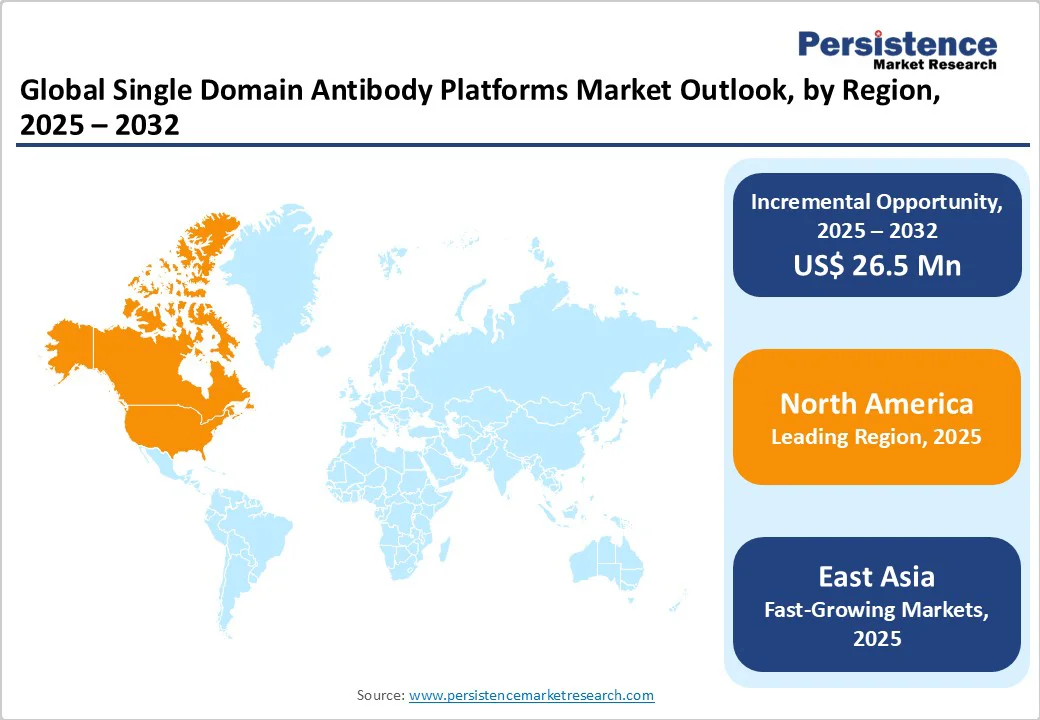
The region is anticipated to dominate with a share of 37.9% in 2025, due to rising number of immunotherapy candidates and clinical trials in North America are driving focus on immuno-oncology, where sdAbs show great promise. Their ability to conjugate with cytotoxic drugs or immune-modulating agents to create Antibody-Drug Conjugates (ADCs) is garnering attention in the cancer treatment space. For instance, The American Society of Clinical Oncology 2022 report found that federal funding provided for cancer research resulted in substantial growth in the diagnosis, treatment, and prevention of cancer. The Congress invested around US$1.25 Bn in funding for cancer research and development, further increasing the funding for the National Institute of Health (NIH), including a US$120 Mn increase in the National Cancer Institute.
Biotechnology and pharmaceutical companies in North America are entering into partnerships with research institutions and biotech firms to develop sdAb-based therapies. Such collaborations are predicted to accelerate the development and commercialization of SdAb products.
Asia Pacific single-domain antibody (sdAb) platforms market is emerging as a dynamic growth hub, fueled by rapid advancements in biotechnology and rising demand for affordable biologics. Countries such as China, India, South Korea, and Japan are investing heavily in research infrastructure, fostering collaborations between academic institutes and biopharma companies to accelerate sdAb innovation. Increasing cancer prevalence, coupled with a high burden of infectious diseases, is driving demand for targeted, patient-friendly therapeutics. Local players are leveraging sdAb’s cost-efficient production and stability to expand into oral formulations and diagnostic applications. Government support, clinical trial expansions, and cross-border partnerships are further positioning Asia–Pacific as a key contributor to global sdAb development, with strong potential for both therapeutic and diagnostic breakthroughs.
Single-domain antibody manufacturers are increasingly concentrating on innovative drug development and securing product patents as strategic measures to maximize market revenue. To strengthen their competitive position, leading players are actively pursuing collaborations with research institutes, biopharma companies, and healthcare providers. These partnerships not only accelerate product innovation but also expand clinical validation, regulatory approvals, and commercialization opportunities.
The global market is projected to be valued at US$ 67.2 Mn in 2025.
Their small size, high tissue penetration, stability, and ability to bind hard-to-reach epitopes make sdAbs more versatile than conventional monoclonal antibodies.
The global market is poised to witness a CAGR of 9.7% between 2025 and 2032.
The ability to conjugate with cytotoxic drugs, radioactive isotopes, and gene-editing tools creates scope for next-generation precision therapeutics.
GenScript Biotech Corporation, Creative Biolab, Synbio Technologies, ProSci Incorporated, and others
| Report Attribute | Details |
|---|---|
|
Historical Data/Actuals |
2019 - 2024 |
|
Forecast Period |
2025 - 2032 |
|
Market Analysis |
Value: US$ Mn |
|
Geographical Coverage |
|
|
Segmental Coverage |
|
|
Competitive Analysis |
|
|
Report Highlights |
|
By Platform Type
By Animal Source
By Application
By End User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author