ID: PMRREP33180| 178 Pages | 9 Sep 2025 | Format: PDF, Excel, PPT* | Healthcare

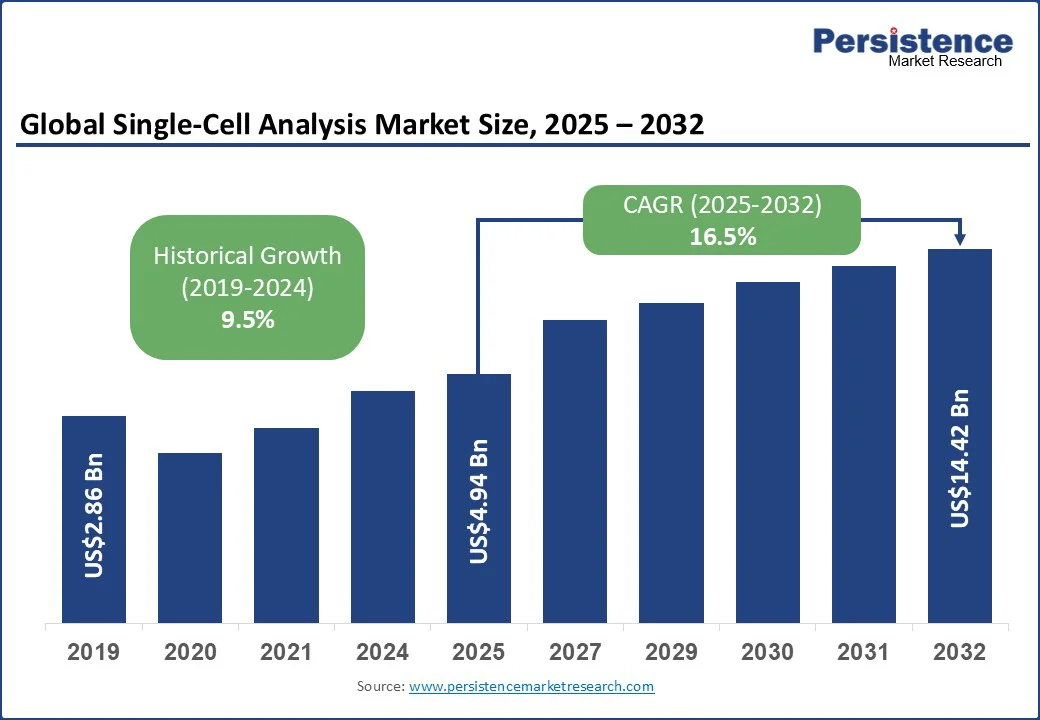
The global single-cell analysis market size is likely to be valued at US$4.94 Bn in 2025, and is estimated to reach US$14.42 Bn by 2032, growing at a CAGR of 16.5% during the forecast period 2025 - 2032.
Key Industry Highlights:
| Global Market Attribute | Key Insights |
|---|---|
| Single-Cell Analysis Market Size (2025E) | US$4.94 Bn |
| Market Value Forecast (2032F) | US$14.42 Bn |
| Projected Growth (CAGR 2025 to 2032) | 16.5% |
| Historical Market Growth (CAGR 2019 to 2024) | 9.5% |
The adoption of single-cell multi-omics and spatial transcriptomics is expanding research frontiers. These technologies help identify rare cell types and cellular states in tumors and tissues, driving market growth. The single-cell analysis market is evolving at a considerable rate, fueled by the rising incidence of cancer and chronic diseases worldwide, which has necessitated an in-depth research into precision diagnostics and targeted therapies.
Single-cell analysis is a cutting-edge scientific technique that dissects biological tissues at the level of individual cells, revealing cellular heterogeneity that bulk analysis methods mask. It enables precise investigation of gene expression, protein levels, and DNA variations on a cell-by-cell basis, thus unveiling information about complex biological processes, disease progression, and therapeutic responses.
This granular approach is crucial for advancing understanding in fields such as cancer genomics, stem cell research, immunology, and personalized medicine. Increasing investments in stem cell research and regenerative medicine, along with advances in next-generation sequencing, microfluidics, and multi-omics integration, are driving technological innovation, enabling high-throughput and spatially resolved single-cell studies.
A key driver of market growth is the rising demand for precision and personalized medicine, an approach that tailors treatment based on a patient's genetic, proteomic, and cellular profile. Single-cell analysis uniquely suits the dynamics of this space, as it involves the dissection of cellular heterogeneity at an unprecedented resolution, enabling clinicians to identify rare tumor clones that are resistant to therapy and tailor immunotherapies accordingly.
The potential of precision medicine and its alignment with single-cell analysis techniques in treating a wide range of conditions, especially rare diseases, has found strong regulatory support as well. For example, in 2023, the U.S. FDA’s Center for Drug Evaluation and Research (CDER) approved 55 novel drugs, of which 28 were greenlit as orphan drugs to prevent, diagnose, or treat rare conditions or diseases.
These approvals signal a growing need for the availability of next-generation therapies for treating or managing life-threatening or life-deteriorating diseases, with approaches based on detailed cellular insights facilitated by single-cell sequencing and spatial transcriptomics technologies.
Cutting-edge technologies such as next-generation sequencing, microfluidics, and multi-omics integration require substantial capital investment upfront for acquisition and maintenance, often exceeding US$500,000 per instrument. Per-sample reagent costs can run into hundreds of dollars, creating a significant financial barrier for many laboratories to engage in single-cell analysis R&D.
Moreover, the complex workflows require a high level of expertise in bioinformatics and specialized infrastructure to manage and interpret large-scale, multidimensional datasets. This skill gap delays data standardization and workflow replicability and productivity, as even well-funded institutions report turnarounds of weeks for analysis.
For instance, a 2024 survey of biotech startups revealed that over 40% cited cost and data analysis challenges as primary impediments to scaling single-cell experiments. As a result, prohibitive costs and the inherent technical complexities of advanced single-cell platforms are likely to severely limit their adoption, especially in emerging economies and smaller research settings.
The convergence of multi-omics integration with AI-powered bioinformatics has led to the building of a transformative platform that has the ability to produce unprecedented cellular insights, unlocking lucrative opportunities in the single-cell analysis market.
Unlike traditional single-parameter analyses, the single-cell multi-omics approach, combining genomics, transcriptomics, proteomics, and epigenomics, enables a holistic view of cellular functions and interactions within complex tissues. This integration is further enhanced by AI and machine learning algorithms, which efficiently process vast, high-dimensional datasets to identify rare cell types, predict therapeutic targets, and personalize treatment regimens.
For example, Illumina's Innovation Roadmap outlines its push to deliver future-ready next-generation and multiomics sequencing technologies, highlighting simplified workflows such as the 2026-bound constellation mapped reads, which streamlines library prep and enhances long-range genomic mapping for deeper biological insight. Furthermore, growing investments in spatial transcriptomics and single-cell epigenetic profiling indicate the expanding breadth of single-cell research beyond oncology into neuroscience, immunology, and regenerative medicine.
For instance, in April 2025, the University of Alabama at Birmingham (UAB) announced US$160,000 in research vouchers to eight teams to support experiments using its new Lunaphore COMET multiplex immunofluorescent platform, enabling automated detection of up to 40 protein markers in a tissue sample and deepening spatial biology insights.
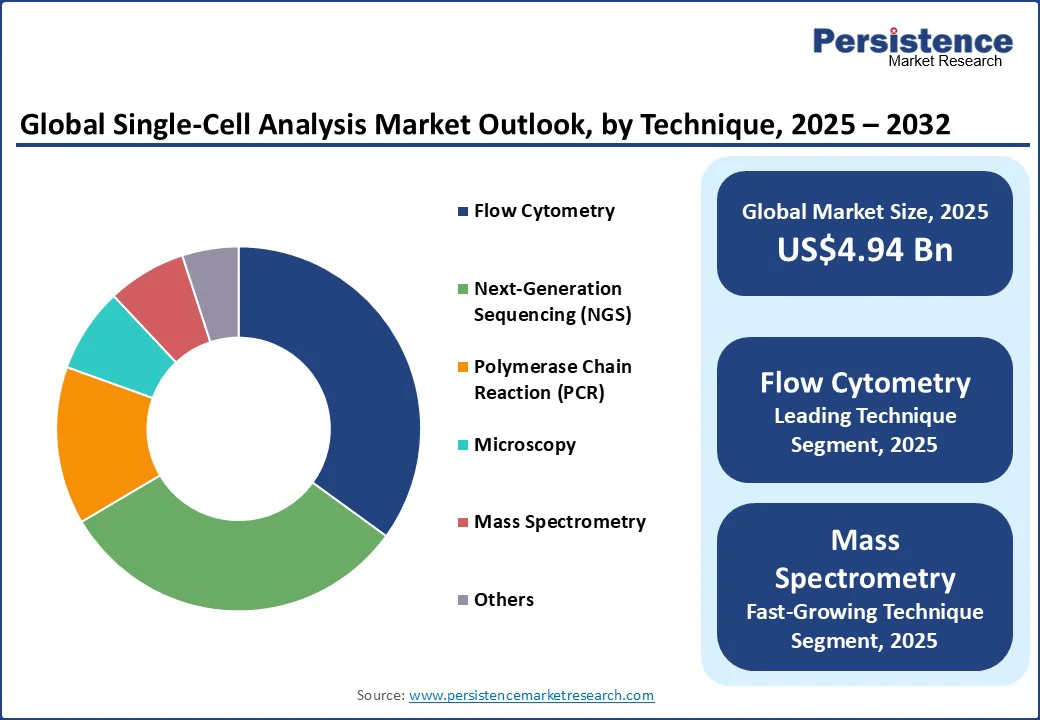
Flow cytometry is anticipated to be the dominant technique segment, holding the largest revenue share of approximately 35% in the single-cell analysis market in 2025. This technique is at the forefront of the market due to its rapid, high-throughput capability to analyze physical and chemical characteristics of thousands of cells per second with high precision.
It is especially critical in oncology, immunology, and cellular therapy development, where phenotypic and functional cellular profiling is foundational. Innovations such as multicolor flow cytometry have expanded its multiplexing capacity, further solidifying its leadership position.
Mass spectrometry is expected to register a soaring CAGR during 2025-2032, owing to the unique capability of this technique to analyze proteomes and metabolomes directly, which sequencing-based approaches cannot fully capture. The shift toward single-cell proteomics is paradigmatic, where functional protein landscapes are mapped to understand cellular behaviors in disease states, drug responses, and tissue microenvironments.
The recent introduction of next-gen instruments with enhanced ion mobility and sensitivity enables routine single-cell protein quantification, resolving an unmet healthcare need. Driven by pressing demands in biomarker discovery and targeted therapeutics, the role of mass spectrometry is rapidly extending into translational and clinical applications.
The cancer research segment is anticipated to lead the market with an estimated revenue share of 32.1% in 2025. The dominance of this segment is rooted in the critical need to understand tumor heterogeneity and the tumor microenvironment intricately at a cellular level, which directly impacts therapeutic success.
Single-cell technologies enable the identification of rare tumor subpopulations and immune cells that contribute to drug resistance, aiding in the design of precision oncology strategies. For example, breakthroughs in single-cell spatial transcriptomics have allowed researchers to map cell-to-cell interactions in breast and lung cancer, helping in the design of more precise immunotherapy regimens.
The immunology segment is set to exhibit the highest CAGR through 2032. The segment growth reflects the widening application of single-cell analysis in dissecting complex immune cell populations and understanding autoimmune diseases, vaccine responses, and infectious disease mechanisms.
The COVID-19 pandemic put the spotlight on the importance of single-cell immunophenotyping in tracking immune responses and vaccine efficacy, resulting in rapid technology adoption and increased R&D investments in immunology-focused single-cell platforms. Advanced immune profiling coupled with multi-omics integration is steering novel therapeutic breakthroughs in areas such as CAR-T cell therapies and checkpoint inhibitors, expanding the immunology market aggressively.
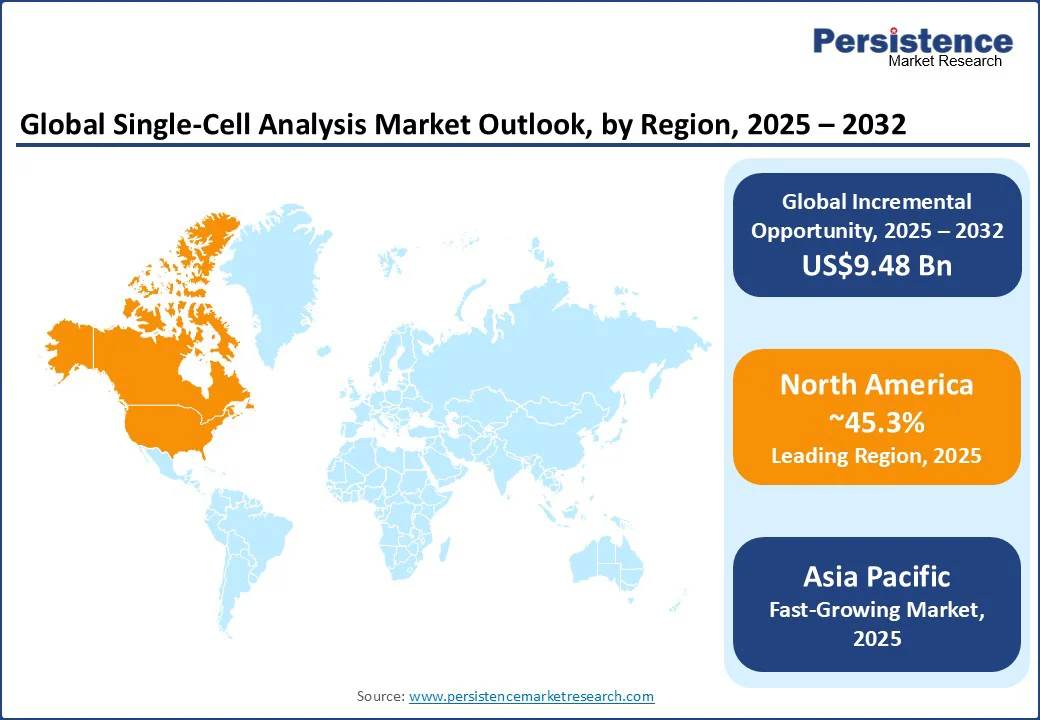
The dominant position of North America, with approximately 45.3% single-cell analysis market share in 2025, is underpinned by its advanced research infrastructure, robust public and private funding for scientific endeavors, and the presence of numerous biotechnology and pharmaceutical giants. The U.S. leads the regional market on the back of extensive adoption of next-generation sequencing technologies and a solid ecosystem of academic and clinical research institutions focused on precision medicine and genomics.
Government initiatives such as the “All of Us” research program of the National Institutes of Health and heavy investments in cancer genomics, immunology, and stem cell research have fostered innovation pipelines and expedited clinical trials. Favorable regulatory frameworks and collaborations between academia and industry further sped up the market entry of novel drugs and therapies in the region.
The Asia Pacific market is expected to exhibit the highest CAGR from 2025 to 2032, driven by expanding budgetary allocation for genomic and life sciences R&D, particularly in China and India. The rising demand for personalized medicine, a strong pharmaceutical manufacturing base, and increasing healthcare infrastructure investments are likely to elevate the demand for high-throughput single-cell analysis technologies.
India is projected to experience the highest regional CAGR due to its growing biotech sector and increasing outsourcing of drug discovery services. The demographic advantages of the region, such as a large pool of untapped patient populations and a growing incidence of chronic diseases, will also generate huge demand for targeted therapies and diagnostics in the forthcoming years.
Supported by a deep-rooted tradition of high-quality biomedical research and established innovation ecosystems in countries such as Germany, the U.K., and France, Europe occupies a central position in the single-cell analysis market. The regional market will also benefit from significant public research funding by the European Union (EU), pan-European collaborations such as Horizon Europe, and an advancing biopharma sector increasingly focused on cell-based therapies and precision therapies.
Consistent regulatory backing has also encouraged translational research in single-cell spatial transcriptomics and multi-omics. In April 2025, for instance, researchers at the Max Delbrück Center used cutting-edge 3D spatial transcriptomics combined with extracellular matrix imaging to map cellular neighborhoods within an early-stage non-small-cell lung tumor, revealing cell niches for drug administration that could inform personalized treatment strategies.
The global single-cell analysis market landscape is a product of technological innovation, strategic collaborations, and diversification of application areas, creating intense competition among key players aiming for leadership. The growing focus on personalized medicine and the need to decode cellular heterogeneity have pushed companies to aggressively expand their portfolios, particularly in multi-omics integration, spatial transcriptomics, and AI-empowered bioinformatics.
High throughput and automation capabilities are also garnering private sector attention, with firms such as BD pioneering robotics-compatible reagent kits to boost efficiency and reproducibility in single-cell workflows. Furthermore, in a bid to differentiate their products, industry leaders are leaning on geographic expansion, targeting high-growth markets by forming local partnerships and investing in scalable instrumentation suites tailored for diverse research needs.
The single-cell analysis market is projected to reach US$4.94 Bn in 2025.
The accelerating demand and adoption of precision and personalized medicine are driving the market.
The market is poised to witness a CAGR of 16.5% from 2025 to 2032.
The convergence of multi-omics integration with AI-powered bioinformatics and growing investments in spatial transcriptomics and single-cell epigenetic profiling are key market opportunities.
10x Genomics, Inc., BD (Becton, Dickinson and Company), and Illumina, Inc. are some of the leading market players.
| Report Attribute | Details |
|---|---|
| Historical Data/Actuals | 2019 - 2024 |
| Forecast Period | 2025 - 2032 |
| Market Analysis | Value: US$ Bn |
| Geographical Coverage |
|
| Segmental Coverage |
|
| Competitive Analysis |
|
| Report Highlights |
|
By Product Type
By Technique
By Application
By End-User
By Region
Delivery Timelines
For more information on this report and its delivery timelines please get in touch with our sales team.
About Author